Integrating the 40-Gene Expression Profile (40-GEP) Test Improves Metastatic Risk-Stratification Within Clinically Relevant Subgroups of High-Risk Cutaneous Squamous Cell Carcinoma (cSCC) Patients
- PMID: 38424384
- PMCID: PMC10965857
- DOI: 10.1007/s13555-024-01111-5
Integrating the 40-Gene Expression Profile (40-GEP) Test Improves Metastatic Risk-Stratification Within Clinically Relevant Subgroups of High-Risk Cutaneous Squamous Cell Carcinoma (cSCC) Patients
Abstract
Introduction: The validated 40-gene expression profile (40-GEP) test independently stratifies risk of regional or distant metastasis for cutaneous squamous cell carcinoma (cSCC) tumors with high-risk clinicopathologic features. This study evaluated the stratification of risk by the 40-GEP test in a large cohort of tumors with one or more high-risk factors and in clinically relevant subgroups, including tumors within National Comprehensive Cancer Network (NCCN) high- and very-high-risk groups, lower-stage BWH T1 and T2a tumors, and patients > 65 years old.
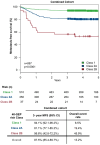
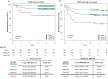
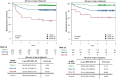
Methods: This multicenter (n = 58) performance study of the 40-GEP included 897 patients. Kaplan-Meier analyses were performed to assess risk stratification profiles for 40-GEP Class 1 (low), Class 2A (higher) and Class 2B (highest) risk groups, while nested Cox regression models were used to compare risk prediction of clinicopathologic risk classification systems versus risk classification systems in combination with 40-GEP.
Results: Patients classified as 40-GEP Class 1, Class 2A, or Class 2B had significantly different metastatic risk profiles (p < 0.0001). Integrating 40-GEP results into models with individual clinicopathologic risk factors or risk classification systems (Brigham and Women's Hospital, American Joint Committee on Cancer Staging Manual, 8th Edition) and NCCN demonstrated significant improvement in accuracy for prediction of metastatic events (ANOVA for model deviance, p < 0.0001 for all models).
Conclusion: The 40-GEP test demonstrates accurate, independent, clinically actionable stratification of metastatic risk and improves predictive accuracy when integrated into risk classification systems. The improved accuracy of risk assessment when including tumor biology via the 40-GEP test ensures more risk-aligned, personalized patient management decisions.
Keywords: 40-GEP; Cutaneous squamous cell carcinoma; Metastasis; Prognostic; Risk assessment.
© 2024. The Author(s).
Conflict of interest statement
Ashley Wysong and Anna Bar receive research funding from CBI. Christie Regula, Ally-Khan Somani, and Sherrif Ibrahim are speakers and principal investigators for CBI. Aaron Farberg is consultant for CBI and has funding from Regeneron, Replimune, Enspectra Health, Gerson Lehrman Group. Alison Fitzgerald, Jennifer Siegel, Anesh Prasai, and Matthew Goldberg are employees, stock and options holders for CBI. Shlomo Koyfman is a consultant for CBI, Merck, BMS, Regeneron and Galera therapeutics; has research support from Merk, BMS and Regeron and honoraria from UpToDate and Varian. Sarah Arron is a paid consultant for Enspectra Health, CBI, and WorldCare Clinical; paid speaker bureaus for Regeneron and CBI; paid expert testimony from Forensis, Inc, and Muro & Lampe; travel expenses paid by CBI; paid participation on a data safety monitoring board for Replimune; paid advisory board participation for Regeneron, Dermatology Times/Multimedia Medical, and Matrix Medical; leadership in the International Transplant Skin Cancer Collaborative; stock holding in Rakuten Medical and Enspectra Health, with stock held by spouse in Genentech and 23andMe. All remaining authors participated as investigators for CBI during this study.
Figures

Similar articles
-
Gene expression profiling for metastatic risk in head and neck cutaneous squamous cell carcinoma.Laryngoscope Investig Otolaryngol. 2022 Jan 6;7(1):135-144. doi: 10.1002/lio2.724. eCollection 2022 Feb. Laryngoscope Investig Otolaryngol. 2022. PMID: 35155791 Free PMC article.
-
The Prognostic Value and Clinical Utility of the 40-Gene Expression Profile (40-GEP) Test in Cutaneous Squamous Cell Carcinoma: Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Apr 25;15(9):2456. doi: 10.3390/cancers15092456. Cancers (Basel). 2023. PMID: 37173922 Free PMC article. Review.
-
Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: impact on patient management.Curr Med Res Opin. 2020 Aug;36(8):1301-1307. doi: 10.1080/03007995.2020.1763284. Epub 2020 May 18. Curr Med Res Opin. 2020. PMID: 32351136
-
Integrating 40-GEP Testing to Improve Clinical Recommendations for Adjuvant Radiation for Cutaneous Squamous Cell Carcinoma: Multidisciplinary Consensus Guidelines.J Clin Aesthet Dermatol. 2024 Mar;17(3 Suppl 2):S3-S8. J Clin Aesthet Dermatol. 2024. PMID: 38495846 Free PMC article. Review.
-
Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test.Future Oncol. 2022 Mar;18(7):833-847. doi: 10.2217/fon-2021-1277. Epub 2021 Nov 25. Future Oncol. 2022. PMID: 34821148
Cited by
-
The Role of Gene Expression Profiling in the Management of Cutaneous Squamous Cell Cancer: A Review.Cancers (Basel). 2024 Nov 23;16(23):3925. doi: 10.3390/cancers16233925. Cancers (Basel). 2024. PMID: 39682114 Free PMC article. Review.
-
Predicting adjuvant radiation therapy benefit in cutaneous squamous cell carcinoma with the 40-gene expression profile.Future Oncol. 2024;20(35):2737-2746. doi: 10.1080/14796694.2024.2390820. Epub 2024 Sep 4. Future Oncol. 2024. PMID: 39229801 Free PMC article.
-
Risk Factor Number and Recurrence, Metastasis, and Disease-Related Death in Cutaneous Squamous Cell Carcinoma.JAMA Dermatol. 2025 Jun 1;161(6):597-604. doi: 10.1001/jamadermatol.2025.0128. JAMA Dermatol. 2025. PMID: 40105853
-
PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature.Biomedicines. 2025 Jan 7;13(1):123. doi: 10.3390/biomedicines13010123. Biomedicines. 2025. PMID: 39857707 Free PMC article. Review.
-
Inconsistent Associations Between Risk Factor Profiles and Adjuvant Radiation Therapy (ART) Treatment in Patients with Cutaneous Squamous Cell Carcinoma and Utility of the 40-Gene Expression Profile to Refine ART Guidance.Dermatol Ther (Heidelb). 2024 Apr;14(4):861-873. doi: 10.1007/s13555-024-01125-z. Epub 2024 Mar 23. Dermatol Ther (Heidelb). 2024. PMID: 38521873 Free PMC article.
References
-
- Skin Cancer Foundation. Our new approach to a challenging skin cancer statistic. The Skin Cancer Foundation; 2021. https://www.skincancer.org/blog/our-new-approach-to-a-challenging-skin-c.... Accessed June 14, 2021.
-
- Soleymani T, Brodland DG, Arzeno J, Sharon DJ, Zitelli JA. Clinical outcomes of high-risk cutaneous squamous cell carcinomas treated with Mohs surgery alone: an analysis of local recurrence, regional nodal metastases, progression-free survival, and disease-specific death. J Am Acad Dermatol. 2023;88(1):109–117. doi: 10.1016/j.jaad.2022.06.1169. - DOI - PubMed
LinkOut - more resources
Full Text Sources

