Interpreting drug synergy in breast cancer with deep learning using target-protein inhibition profiles
- PMID: 38424554
- PMCID: PMC10905801
- DOI: 10.1186/s13040-024-00359-z
Interpreting drug synergy in breast cancer with deep learning using target-protein inhibition profiles
Abstract
Background: Breast cancer is the most common malignancy among women worldwide. Despite advances in treating breast cancer over the past decades, drug resistance and adverse effects remain challenging. Recent therapeutic progress has shifted toward using drug combinations for better treatment efficiency. However, with a growing number of potential small-molecule cancer inhibitors, in silico strategies to predict pharmacological synergy before experimental trials are required to compensate for time and cost restrictions. Many deep learning models have been previously proposed to predict the synergistic effects of drug combinations with high performance. However, these models heavily relied on a large number of drug chemical structural fingerprints as their main features, which made model interpretation a challenge.
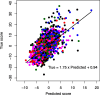
Results: This study developed a deep neural network model that predicts synergy between small-molecule pairs based on their inhibitory activities against 13 selected key proteins. The synergy prediction model achieved a Pearson correlation coefficient between model predictions and experimental data of 0.63 across five breast cancer cell lines. BT-549 and MCF-7 achieved the highest correlation of 0.67 when considering individual cell lines. Despite achieving a moderate correlation compared to previous deep learning models, our model offers a distinctive advantage in terms of interpretability. Using the inhibitory activities against key protein targets as the main features allowed a straightforward interpretation of the model since the individual features had direct biological meaning. By tracing the synergistic interactions of compounds through their target proteins, we gained insights into the patterns our model recognized as indicative of synergistic effects.
Conclusions: The framework employed in the present study lays the groundwork for future advancements, especially in model interpretation. By combining deep learning techniques and target-specific models, this study shed light on potential patterns of target-protein inhibition profiles that could be exploited in breast cancer treatment.
Keywords: Deep neural network; Drug combination; Small-molecule inhibitors; Synergistic effects; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Grants and funding
- FRB660073/0164 (Program Sustainable Bioeconomy)/Thailand Science Research and Innovation (TSRI) Basic Research Fund: The fiscal year 2023
- FRB660073/0164 (Program Sustainable Bioeconomy)/Thailand Science Research and Innovation (TSRI) Basic Research Fund: The fiscal year 2023
- FRB660073/0164 (Program Sustainable Bioeconomy)/Thailand Science Research and Innovation (TSRI) Basic Research Fund: The fiscal year 2023
LinkOut - more resources
Full Text Sources
Miscellaneous

