Enhanced bacterial cellulose production in Komagataeibacter sucrofermentans: impact of different PQQ-dependent dehydrogenase knockouts and ethanol supplementation
- PMID: 38424558
- PMCID: PMC10902950
- DOI: 10.1186/s13068-024-02482-9
Enhanced bacterial cellulose production in Komagataeibacter sucrofermentans: impact of different PQQ-dependent dehydrogenase knockouts and ethanol supplementation
Abstract
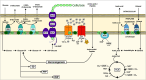
Background: Bacterial cellulose (BC) is a biocompatible material with unique mechanical properties, thus holding a significant industrial potential. Despite many acetic acid bacteria (AAB) being BC overproducers, cost-effective production remains a challenge. The role of pyrroloquinoline quinone (PQQ)-dependent membrane dehydrogenases (mDH) is crucial in the metabolism of AAB since it links substrate incomplete oxidation in the periplasm to energy generation. Specifically, glucose oxidation to gluconic acid substantially lowers environmental pH and hinders BC production. Conversely, ethanol supplementation is known to enhance BC yields in Komagataeibacter spp. by promoting efficient glucose utilization.
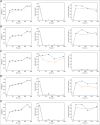
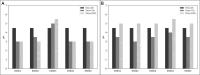
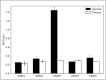
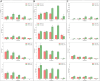
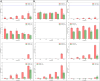
Results: K. sucrofermentans ATCC 700178 was engineered, knocking out the four PQQ-mDHs, to assess their impact on BC production. The strain KS003, lacking PQQ-dependent glucose dehydrogenase (PQQ-GDH), did not produce gluconic acid and exhibited a 5.77-fold increase in BC production with glucose as the sole carbon source, and a 2.26-fold increase under optimal ethanol supplementation conditions. In contrast, the strain KS004, deficient in the PQQ-dependent alcohol dehydrogenase (PQQ-ADH), showed no significant change in BC yield in the single carbon source experiment but showed a restrained benefit from ethanol supplementation.
Conclusions: The results underscore the critical influence of PQQ-GDH and PQQ-ADH and clarify the effect of ethanol supplementation on BC production in K. sucrofermentans ATCC 700178. This study provides a foundation for further metabolic pathway optimization, emphasizing the importance of diauxic ethanol metabolism for high BC production.
Keywords: Komagataeibacter; Acetic acid; Acetic acid bacteria; Bacterial cellulose; Gluconic acid; Metabolic engineering; PQQ-dependent dehydrogenases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Cellulose production from glucose using a glucose dehydrogenase gene (gdh)-deficient mutant of Gluconacetobacter xylinus and its use for bioconversion of sweet potato pulp.J Biosci Bioeng. 2005 Apr;99(4):415-22. doi: 10.1263/jbb.99.415. J Biosci Bioeng. 2005. PMID: 16233811
-
Regulation of Pyrroloquinoline Quinone-Dependent Glucose Dehydrogenase Activity in the Model Rhizosphere-Dwelling Bacterium Pseudomonas putida KT2440.Appl Environ Microbiol. 2016 Jul 29;82(16):4955-64. doi: 10.1128/AEM.00813-16. Print 2016 Aug 15. Appl Environ Microbiol. 2016. PMID: 27287323 Free PMC article.
-
Engineering CRISPR interference system to enhance the production of pyrroloquinoline quinone in Klebsiella pneumonia.Lett Appl Microbiol. 2020 Sep;71(3):242-250. doi: 10.1111/lam.13311. Epub 2020 Jun 4. Lett Appl Microbiol. 2020. PMID: 32394472
-
Pyrroloquinoline quinone-dependent dehydrogenases of acetic acid bacteria.Appl Microbiol Biotechnol. 2018 Nov;102(22):9531-9540. doi: 10.1007/s00253-018-9360-3. Epub 2018 Sep 15. Appl Microbiol Biotechnol. 2018. PMID: 30218379 Review.
-
The biochemistry, physiology and genetics of PQQ and PQQ-containing enzymes.Adv Microb Physiol. 1998;40:1-80. doi: 10.1016/s0065-2911(08)60129-0. Adv Microb Physiol. 1998. PMID: 9889976 Review.
Cited by
-
How carbon sources drive cellulose synthesis in two Komagataeibacter xylinus strains.Sci Rep. 2024 Sep 3;14(1):20494. doi: 10.1038/s41598-024-71648-0. Sci Rep. 2024. PMID: 39227724 Free PMC article.
-
Modulating Microbial Materials - Engineering Bacterial Cellulose with Synthetic Biology.ACS Synth Biol. 2024 Dec 20;13(12):3857-3875. doi: 10.1021/acssynbio.4c00615. Epub 2024 Nov 7. ACS Synth Biol. 2024. PMID: 39509658 Free PMC article. Review.
-
Bacterial Cellulose for Scalable and Sustainable Bio-Gels in the Circular Economy.Gels. 2025 Apr 2;11(4):262. doi: 10.3390/gels11040262. Gels. 2025. PMID: 40277698 Free PMC article. Review.
-
Construction of a synthetic biology toolkit for the genetic manipulation in the cellulose-producing strain Kosakonia oryzendophytica.Synth Syst Biotechnol. 2025 May 27;10(3):1050-1058. doi: 10.1016/j.synbio.2025.05.012. eCollection 2025 Sep. Synth Syst Biotechnol. 2025. PMID: 40529626 Free PMC article.
References
-
- Gullo M, La China S, Falcone PM, Giudici P. Biotechnological production of cellulose by acetic acid bacteria: current state and perspectives. Appl Microbiol Biotechnol. 2018;102:6885–6898. - PubMed
-
- Lin D, Lopez-Sanchez P, Li R, Li Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour Technol. 2014;151:113–119. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
