The SGLT2 inhibitor Empagliflozin promotes post-stroke functional recovery in diabetic mice
- PMID: 38424560
- PMCID: PMC10905950
- DOI: 10.1186/s12933-024-02174-6
The SGLT2 inhibitor Empagliflozin promotes post-stroke functional recovery in diabetic mice
Abstract
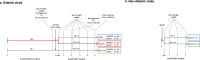
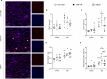
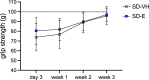
Type-2 diabetes (T2D) worsens stroke recovery, amplifying post-stroke disabilities. Currently, there are no therapies targeting this important clinical problem. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are potent anti-diabetic drugs that also efficiently reduce cardiovascular death and heart failure. In addition, SGLT2i facilitate several processes implicated in stroke recovery. However, the potential efficacy of SGLT2i to improve stroke recovery in T2D has not been investigated. Therefore, we determined whether a post-stroke intervention with the SGLT2i Empagliflozin could improve stroke recovery in T2D mice. T2D was induced in C57BL6J mice by 8 months of high-fat diet feeding. Hereafter, animals were subjected to transient middle cerebral artery occlusion and treated with vehicle or the SGLTi Empagliflozin (10 mg/kg/day) starting from 3 days after stroke. A similar study in non diabetic mice was also conducted. Stroke recovery was assessed using the forepaw grip strength test. To identify potential mechanisms involved in the Empagliflozin-mediated effects, several metabolic parameters were assessed. Additionally, neuronal survival, neuroinflammation, neurogenesis and cerebral vascularization were analyzed using immunohistochemistry/quantitative microscopy. Empagliflozin significantly improved stroke recovery in T2D but not in non-diabetic mice. Improvement of functional recovery was associated with lowered glycemia, increased serum levels of fibroblast growth factor-21 (FGF-21), and the normalization of T2D-induced aberration of parenchymal pericyte density. The global T2D-epidemic and the fact that T2D is a major risk factor for stroke are drastically increasing the number of people in need of efficacious therapies to improve stroke recovery. Our data provide a strong incentive for the potential use of SGLT2i for the treatment of post-stroke sequelae in T2D.
Keywords: Diabetes; Empagliflozin; Mouse; Sodium-glucose cotransporter 2 inhibitors; Stroke recovery.
© 2024. The Author(s).
Conflict of interest statement
This study was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. TK is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. T.N. has received unrestricted grants from AstraZeneca and consultancy fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Merck and Sanofi-Aventis. The other authors declare that they have no competing interests.
Figures




References
-
- Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project. Stroke. 2003;34(3):688–694. doi: 10.1161/01.STR.0000057975.15221.40. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

