Cost-of-illness studies of inherited retinal diseases: a systematic review
- PMID: 38424595
- PMCID: PMC10905859
- DOI: 10.1186/s13023-024-03099-9
Cost-of-illness studies of inherited retinal diseases: a systematic review
Abstract
Background: While health care and societal costs are routinely modelled for most diseases, there is a paucity of comprehensive data and cost-of-illness (COI) studies for inherited retinal diseases (IRDs). This lack of data can lead to underfunding or misallocation of resources. A comprehensive understanding of the COI of IRDs would assist governmental and healthcare leaders in determining optimal resource allocation, prioritizing funding for research, treatment, and support services for these patients.
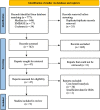
Methods: Following PRISMA guidelines, a literature search was conducted using Medline, EMBASE and Cochrane databases, from database inception up to 30 Jun 2023, to identify COI studies related to IRD. Original studies in English, primarily including patients with IRDs, and whose main study objective was the estimation of the costs of IRDs and had sufficiently detailed methodology to assess study quality were eligible for inclusion. To enable comparison across countries and studies, all annual costs were standardized to US dollars, adjusted for inflation to reflect their current value and recalculated on a "per patient" basis wherever possible. The review protocol was registered in PROSPERO (registration number CRD42023452986).
Results: A total of nine studies were included in the final stage of systematic review and they consistently demonstrated a significant disease burden associated with IRDs. In Singapore, the mean total cost per patient was roughly US$6926/year. In Japan, the mean total cost per patient was US$20,833/year. In the UK, the mean total cost per patient with IRD ranged from US$21,658 to US$36,549/year. In contrast, in the US, the mean total per-patient costs for IRDs ranged from about US$33,017 to US$186,051 per year. In Canada, these mean total per-patient costs varied between US$16,470 and US$275,045/year. Non-health costs constituted the overwhelming majority of costs as compared to healthcare costs; 87-98% of the total costs were due to non-health costs, which could be attributed to diminished quality of life, poverty, and increased informal caregiving needs for affected individuals.
Conclusion: IRDs impose a disproportionate societal burden outside health systems. It is vital for continued funding into IRD research, and governments should incorporate societal costs in the evaluation of cost-effectiveness for forthcoming IRD interventions, including genomic testing and targeted therapies.
Keywords: Blindness; Cost-of-illness; Health economics; Inherited retinal disease; Retinitis pigmentosa.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
Cited by
-
The effectiveness and value of sonpiretigene isteparvovec for the treatment of advanced retinitis pigmentosa.J Manag Care Spec Pharm. 2025 Aug;31(8):835-840. doi: 10.18553/jmcp.2025.31.8.835. J Manag Care Spec Pharm. 2025. PMID: 40704855 Free PMC article. No abstract available.
-
"I miss stars, too": a thematic analysis of the experiences of persons with retinitis pigmentosa using Reddit.J Community Genet. 2025 Apr 29. doi: 10.1007/s12687-025-00796-1. Online ahead of print. J Community Genet. 2025. PMID: 40299283
-
Comparative policy analysis of national rare disease funding policies in Australia, Singapore, South Korea, the United Kingdom and the United States: a scoping review.Health Econ Rev. 2024 Jun 19;14(1):42. doi: 10.1186/s13561-024-00519-1. Health Econ Rev. 2024. PMID: 38896399 Free PMC article.
-
Real-World Research on Retinal Diseases Using Health Claims Database: A Narrative Review.Diagnostics (Basel). 2024 Jul 19;14(14):1568. doi: 10.3390/diagnostics14141568. Diagnostics (Basel). 2024. PMID: 39061705 Free PMC article. Review.
References
-
- Van Schil K, Naessens S, Van de Sompele S, Carron M, Aslanidis A, Van Cauwenbergh C, Kathrin Mayer A, Van Heetvelde M, Bauwens M, Verdin H, Coppieters F, Greenberg ME, Yang MG, Karlstetter M, Langmann T, De Preter K, Kohl S, Cherry TJ, Leroy BP; CNV Study Group; De Baere E. Mapping the genomic landscape of inherited retinal disease genes prioritizes genes prone to coding and noncoding copy-number variations. Genet Med. 2018;20(2):202–213. 10.1038/gim.2017.97. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous