Deep learning segmentation of the choroid plexus from structural magnetic resonance imaging (MRI): validation and normative ranges across the adult lifespan
- PMID: 38424598
- PMCID: PMC10903155
- DOI: 10.1186/s12987-024-00525-9
Deep learning segmentation of the choroid plexus from structural magnetic resonance imaging (MRI): validation and normative ranges across the adult lifespan
Abstract
Background: The choroid plexus functions as the blood-cerebrospinal fluid (CSF) barrier, plays an important role in CSF production and circulation, and has gained increased attention in light of the recent elucidation of CSF circulation dysfunction in neurodegenerative conditions. However, methods for routinely quantifying choroid plexus volume are suboptimal and require technical improvements and validation. Here, we propose three deep learning models that can segment the choroid plexus from commonly-acquired anatomical MRI data and report performance metrics and changes across the adult lifespan.
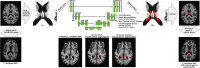
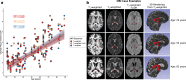
Methods: Fully convolutional neural networks were trained from 3D T1-weighted, 3D T2-weighted, and 2D T2-weighted FLAIR MRI using gold-standard manual segmentations in control and neurodegenerative participants across the lifespan (n = 50; age = 21-85 years). Dice coefficients, 95% Hausdorff distances, and area-under-curve (AUCs) were calculated for each model and compared to segmentations from FreeSurfer using two-tailed Wilcoxon tests (significance criteria: p < 0.05 after false discovery rate multiple comparisons correction). Metrics were regressed against lateral ventricular volume using generalized linear models to assess model performance for varying levels of atrophy. Finally, models were applied to an expanded cohort of adult controls (n = 98; age = 21-89 years) to provide an exemplar of choroid plexus volumetry values across the lifespan.
Results: Deep learning results yielded Dice coefficient = 0.72, Hausdorff distance = 1.97 mm, AUC = 0.87 for T1-weighted MRI, Dice coefficient = 0.72, Hausdorff distance = 2.22 mm, AUC = 0.87 for T2-weighted MRI, and Dice coefficient = 0.74, Hausdorff distance = 1.69 mm, AUC = 0.87 for T2-weighted FLAIR MRI; values did not differ significantly between MRI sequences and were statistically improved compared to current commercially-available algorithms (p < 0.001). The intraclass coefficients were 0.95, 0.95, and 0.96 between T1-weighted and T2-weighted FLAIR, T1-weighted and T2-weighted, and T2-weighted and T2-weighted FLAIR models, respectively. Mean lateral ventricle choroid plexus volume across all participants was 3.20 ± 1.4 cm3; a significant, positive relationship (R2 = 0.54-0.60) was observed between participant age and choroid plexus volume for all MRI sequences (p < 0.001).
Conclusions: Findings support comparable performance in choroid plexus delineation between standard, clinically available, non-contrasted anatomical MRI sequences. The software embedding the evaluated models is freely available online and should provide a useful tool for the growing number of studies that desire to quantitatively evaluate choroid plexus structure and function ( https://github.com/hettk/chp_seg ).
Keywords: Cerebrospinal fluid; Choroid plexus; Deep learning; Glymphatic; Neurofluids; Segmentation.
© 2024. The Author(s).
Conflict of interest statement
No competing interests to declare.
Figures





References
-
- Ricigliano VAG, Morena E, Colombi A, Tonietto M, Hamzaoui M, Poirion E, Bottlaender M, Gervais P, Louapre C, Bodini B, Stankoff B. Choroid plexus enlargement in inflammatory multiple sclerosis: 3.0-T MRI and translocator protein PET evaluation. Radiology. 2021;301(1):166–177. doi: 10.1148/radiol.2021204426. - DOI - PubMed
-
- Yasmin A, Pitkänen A, Andrade P, Paananen T, et al. Post-injury ventricular enlargement associates with iron in choroid plexus but not with seizure susceptibility nor lesion atrophy-6-month MRI follow-up after experimental traumatic brain injury. Brain Struct Funct. 2022;227:145–158. doi: 10.1007/s00429-021-02395-5. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

