Photo-crosslinkable polyester microneedles as sustained drug release systems toward hypertrophic scar treatment
- PMID: 38424728
- PMCID: PMC10956933
- DOI: 10.1080/10717544.2024.2305818
Photo-crosslinkable polyester microneedles as sustained drug release systems toward hypertrophic scar treatment
Abstract
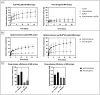
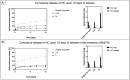
Burn injuries can result in a significant inflammatory response, often leading to hypertrophic scarring (HTS). Local drug therapies e.g. corticoid injections are advised to treat HTS, although they are invasive, operator-dependent, extremely painful and do not permit extended drug release. Polymer-based microneedle (MN) arrays can offer a viable alternative to standard care, while allowing for direct, painless dermal drug delivery with tailorable drug release profile. In the current study, we synthesized photo-crosslinkable, acrylate-endcapped urethane-based poly(ε-caprolactone) (AUP-PCL) toward the fabrication of MNs. Physico-chemical characterization (1H-NMR, evaluation of swelling, gel fraction) of the developed polymer was performed and confirmed successful acrylation of PCL-diol. Subsequently, AUP-PCL, and commercially available PCL-based microneedle arrays were fabricated for comparative evaluation of the constructs. Hydrocortisone was chosen as model drug. To enhance the drug release efficiency of the MNs, Brij®35, a nonionic surfactant was exploited. The thermal properties of the MNs were evaluated via differential scanning calorimetry. Compression testing of the arrays confirmed that the MNs stay intact upon applying a load of 7 N, which correlates to the standard dermal insertion force of MNs. The drug release profile of the arrays was evaluated, suggesting that the developed PCL arrays can offer efficient drug delivery for up to two days, while the AUP-PCL arrays can provide a release up to three weeks. Finally, the insertion of MN arrays into skin samples was performed, followed by histological analysis demonstrating the AUP-PCL MNs outperforming the PCL arrays upon providing pyramidical-shaped perforations through the epidermal layer of the skin.
Keywords: Microneedles; controlled drug release; cortisone; hypertrophic scarring; intradermal drug delivery; polyester.
Plain language summary
AUP-PCL MN arrays provide long-term transdermal drug delivery of hydrocortisoneAUP-PCL-based MN arrays provide superior drug release profiles compared to PCL MNsEffective skin penetration AUP-PCL-based MNs on skin was achieved.
Figures









References
-
- Andersen TE, Andersen AJ, Petersen RS, et al. ( 2018). Drug loaded biodegradable polymer microneedles fabricated by hot embossing. Microelectron Eng 195: 1–16. doi:10.1016/j.mee.2018.03.024. - DOI
-
- Johnson PM, Meinhold KL, Ohl NR, et al. ( 2022). Surfactant molecular properties control location in emulsion electrospun fibers and dictate resulting fiber properties. Macromolecules 55: 9186–95. doi:10.1021/acs.macromol.2c00998. - DOI
-
- Arslan A, Steiger W, Roose P, et al. ( 2021). Polymer architecture as key to unprecedented high-resolution 3D-printing performance: The case of biodegradable hexa-functional telechelic urethane-based poly-ε-caprolactone. Mater Today 44: 25–39. doi:10.1016/j.mattod.2020.10.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
