Vitamin-C-dependent downregulation of the citrate metabolism pathway potentiates pancreatic ductal adenocarcinoma growth arrest
- PMID: 38425123
- PMCID: PMC11467799
- DOI: 10.1002/1878-0261.13616
Vitamin-C-dependent downregulation of the citrate metabolism pathway potentiates pancreatic ductal adenocarcinoma growth arrest
Abstract
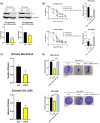
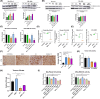
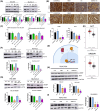
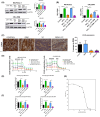
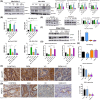
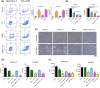
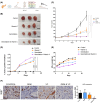
In pancreatic ductal adenocarcinoma (PDAC), metabolic rewiring and resistance to standard therapy are closely associated. PDAC cells show enormous requirements for glucose-derived citrate, the first rate-limiting metabolite in the synthesis of new lipids. Both the expression and activity of citrate synthase (CS) are extraordinarily upregulated in PDAC. However, no previous relationship between gemcitabine response and citrate metabolism has been documented in pancreatic cancer. Here, we report for the first time that pharmacological doses of vitamin C are capable of exerting an inhibitory action on the activity of CS, reducing glucose-derived citrate levels. Moreover, ascorbate targets citrate metabolism towards the de novo lipogenesis pathway, impairing fatty acid synthase (FASN) and ATP citrate lyase (ACLY) expression. Lowered citrate availability was found to be directly associated with diminished proliferation and, remarkably, enhanced gemcitabine response. Moreover, the deregulated citrate-derived lipogenic pathway correlated with a remarkable decrease in extracellular pH through inhibition of lactate dehydrogenase (LDH) and overall reduced glycolytic metabolism. Modulation of citric acid metabolism in highly chemoresistant pancreatic adenocarcinoma, through molecules such as vitamin C, could be considered as a future clinical option to improve patient response to standard chemotherapy regimens.
Keywords: PDAC; citrate synthase; gemcitabine; metabolism; vitamin C.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, et al. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27(4B):2523–2527. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

