Matrix metalloproteinase-8 regulates dendritic cell tolerance in late polymicrobial sepsis via the nuclear factor kappa-B p65/β-catenin pathway
- PMID: 38425412
- PMCID: PMC10903637
- DOI: 10.1093/burnst/tkad025
Matrix metalloproteinase-8 regulates dendritic cell tolerance in late polymicrobial sepsis via the nuclear factor kappa-B p65/β-catenin pathway
Abstract
Background: Tolerogenic dendritic cells (DCs) are associated with poor prognosis of sepsis. Matrix metalloproteinases (MMPs) have been shown to have immunomodulatory effects. However, whether MMPs are involved in the functional reprogramming of DCs is unknown. The study aims to investigate the role of MMPs in sepsis-induced DCs tolerance and the potential mechanisms.
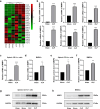
Methods: A murine model of late sepsis was induced by cecal ligation and puncture (CLP). The expression levels of members of the MMP family were detected in sepsis-induced tolerogenic DCs by using microarray assessment. The potential roles and mechanisms underlying MMP8 in the differentiation, maturation and functional reprogramming of DCs during late sepsis were assessed both in vitro and in vivo.
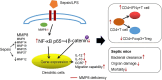
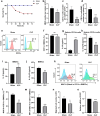
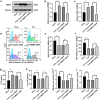
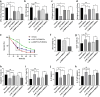
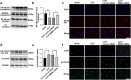
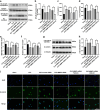
Results: DCs from late septic mice expressed higher levels of MMP8, MMP9, MMP14, MMP19, MMP25 and MMP27, and MMP8 levels were the highest. MMP8 deficiency significantly alleviated sepsis-induced immune tolerance of DCs both in vivo and in vitro. Adoptive transfer of MMP8 knockdown post-septic bone marrow-derived DCs protected mice against sepsis-associated lethality and organ dysfunction, inhibited regulatory T-cell expansion and enhanced Th1 response. Furthermore, the effect of MMP8 on DC tolerance was found to be associated with the nuclear factor kappa-B p65/β-catenin pathway.
Conclusions: Increased MMP8 levels in septic DCs might serve as a negative feedback loop, thereby suppressing the proinflammatory response and inducing DC tolerance.
Keywords: Dendritic cells; Immune tolerance; Matrix metalloproteinases; Nuclear factor kappa-B; Sepsis; Signaling pathway; β-catenin.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures

References
-
- Zhang Y, Chen L, Luo Y, Wang K, Liu X, Xiao Z, et al. . Pink1/parkin-mediated Mitophagy regulated the apoptosis of dendritic cells in sepsis. Inflammation. 2022;45:1374–87. - PubMed
-
- Venet F, Demaret J, Gossez M, Monneret G. Myeloid cells in sepsis-acquired immunodeficiency. Ann N Y Acad Sci. 2021;1499:3–17. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

