Bioactive Protein and Peptide Release from a Mucoadhesive Electrospun Membrane
- PMID: 38425458
- PMCID: PMC10899313
- DOI: 10.1007/s44174-023-00098-5
Bioactive Protein and Peptide Release from a Mucoadhesive Electrospun Membrane
Abstract
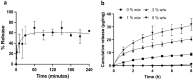
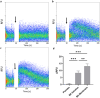
Protein-based biologics constitute a rapidly expanding category of therapeutic agents with high target specificity. Their clinical use has dramatically increased in recent years, but administration is largely via injection. Drug delivery across the oral mucosa is a promising alternative to injections, in order to avoid the gastrointestinal tract and first-pass metabolism. Current drug delivery formulations include liquid sprays, mucoadhesive tablets and films, which lack dose control in the presence of salivary flow. To address this, electrospun membranes that adhere tightly to the oral mucosa and release drugs locally have been developed. Here, we investigated the suitability of these mucoadhesive membranes for peptide or protein release. Bradykinin (0.1%) or insulin (1, 3, and 5%) were incorporated by electrospinning from ethanol/water mixtures. Immersion of membranes in buffer resulted in the rapid release of bradykinin, with a maximal release of 70 ± 12% reached after 1 h. In contrast, insulin was liberated more slowly, with 88 ± 11, 69.0 ± 5.4, and 63.9 ± 9.0% cumulative release of the total encapsulated dose after 8 h for membranes containing 1, 3, and 5% w/w insulin, respectively. Membrane-eluted bradykinin retained pharmacological activity by inducing rapid intracellular calcium release upon binding to its cell surface receptor on oral fibroblasts, when examined by flow cytometry. To quantify further, time-lapse confocal microscopy revealed that membrane-eluted bradykinin caused a 1.58 ± 0.16 fold-change in intracellular calcium fluorescence after 10 s compared to bradykinin solution (2.13 ± 0.21), relative to placebo. In conclusion, these data show that electrospun membranes may be highly effective vehicles for site-specific administration of biotherapeutic proteins or peptides directly to the oral mucosa for either local or systemic drug delivery applications.
Keywords: Bradykinin; Drug delivery; Electrospinning; Insulin; Mucoadhesion; Oral mucosa; Peptides.
© The Author(s) 2023.
Conflict of interest statement
Competing interestJGE, HEC, SGS and CM declare that they have no other known competing financial interests.
Figures



References
-
- Albericio F, Kruger HG. Therapeutic peptides. Fut. Med. Chem. 2012;4(12):1527–1531. - PubMed
-
- Surendranath M, Rekha MR, Parameswaran R. Recent advances in functionally modified polymers for mucoadhesive drug delivery. J. Mater. Chem. B. 2022;10(31):5913–5924. - PubMed
-
- Bernstein G. Delivery of insulin to the buccal mucosa utilizing the RapidMist system. Expert Opin. Drug. Deliv. 2008;5(9):1047–1055. - PubMed
LinkOut - more resources
Full Text Sources
