Borylated cyclobutanes via thermal [2 + 2]-cycloaddition
- PMID: 38425521
- PMCID: PMC10901489
- DOI: 10.1039/d3sc06600b
Borylated cyclobutanes via thermal [2 + 2]-cycloaddition
Abstract
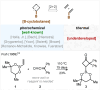
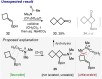
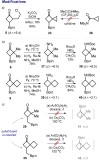
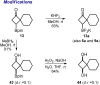
A one-step approach to borylated cyclobutanes from amides of carboxylic acids and vinyl boronates is elaborated. The reaction proceeds via the thermal [2 + 2]-cycloaddition of in situ-generated keteniminium salts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors are employees of a chemical supplier Enamine.
Figures

Similar articles
-
Unexpected Direct Synthesis of N-Vinyl Amides through Vinyl Azide-Enolate [3+2] Cycloaddition.Angew Chem Int Ed Engl. 2017 Jun 19;56(26):7420-7424. doi: 10.1002/anie.201702727. Epub 2017 May 23. Angew Chem Int Ed Engl. 2017. PMID: 28544052
-
Synthesis of beta,beta-disubstituted vinyl boronates via the ruthenium-catalyzed Alder ene reaction of borylated alkynes and alkenes.J Am Chem Soc. 2005 Mar 16;127(10):3252-3. doi: 10.1021/ja0424629. J Am Chem Soc. 2005. PMID: 15755122
-
Synthesis of 3-borylated cyclobutanols from epihalohydrins or epoxy alcohol derivatives.Chem Sci. 2022 Dec 21;14(4):963-969. doi: 10.1039/d2sc06088d. eCollection 2023 Jan 25. Chem Sci. 2022. PMID: 36755731 Free PMC article.
-
Synthesis of 3-Borylated Pyrrolidines by 1,3-Dipolar Cycloaddition of Alkenyl Boronates and Azomethine Ylide.Chemistry. 2022 Sep 27;28(54):e202202117. doi: 10.1002/chem.202202117. Epub 2022 Sep 2. Chemistry. 2022. PMID: 35938353
-
Dual Activation Strategy to Achieve C-C Cleavage of Cyclobutanes: Development and Mechanism of Rh and Zn Cocatalyzed [4 + 2] Cycloaddition of Yne-Vinylcyclobutanones.J Am Chem Soc. 2022 Nov 30;144(47):21457-21469. doi: 10.1021/jacs.2c04244. Epub 2022 Nov 16. J Am Chem Soc. 2022. PMID: 36383143
Cited by
-
Synthesis of α-substituted cyclic boronates via titanium-catalyzed cyclization of vinyl boronates with dihaloalkanes.Chem Sci. 2025 Mar 12;16(15):6515-6521. doi: 10.1039/d5sc01132a. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40103718 Free PMC article.
-
Cu-Catalyzed Chemoselective Borylcupration of Borylated (Z)‑Skipped Dienoates: A Case Study for the Synthesis of gem-diborylcyclobutanes.ACS Catal. 2025 Jul 1;15(14):12063-12074. doi: 10.1021/acscatal.5c02260. eCollection 2025 Jul 18. ACS Catal. 2025. PMID: 40741545 Free PMC article.
References
-
- Chemical structure search drugbank online, https://go.drugbank.com/structures/search/small_molecule_drugs/structure, accessed December 2023, filters used: “substucture”+“approved”+”vet approved”
-
- Armstrong R. J. Aggarwal V. 50 Years of Zweifel Olefination: a Transition-Metal-Free Coupling. Synthesis. 2017;49:3323–3336. doi: 10.1055/s-0036-1589046. - DOI
- Sandford C. Aggarwal V. K. Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun. 2017;53:5481–5494. doi: 10.1039/C7CC01254C. - DOI - PubMed
- Fyfe J. W. B. Watson A. J. B. Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem. 2017;3:31–55. doi: 10.1016/j.chempr.2017.05.008. - DOI
-
- He J. Shao Q. Wu Q. Yu J.-Q. Pd(II)-Catalyzed Enantioselective C(sp3)-H Borylation. J. Am. Chem. Soc. 2017;139:3344–3347. doi: 10.1021/jacs.6b13389. - DOI - PubMed
- Murakami R. Tsunoda K. Iwai T. Sawamura M. Stereoselective C-H Borylations of Cyclopropanes and Cyclobutanes with Silica-Supported Monophosphane-Ir Catalysts. Chem.–Eur. J. 2014;20:13127–13131. doi: 10.1002/chem.201404362. - DOI - PubMed
- Chen X. Chen L. Zhao H. Gao Q. Shen Z. Xu S. Iridium-Catalyzed Enantioselective C(sp3)–H Borylation of Cyclobutanes. Chin. J. Chem. 2020;38:1533–1537. doi: 10.1002/cjoc.202000240. - DOI
- Gao Q. Xu S. Site- and Stereoselective C(sp3)−H Borylation of Strained (Hetero)Cycloalkanols Enabled by Iridium Catalysis. Angew. Chem., Int. Ed. 2023;62:e202218025. doi: 10.1002/anie.202218025. - DOI - PubMed
LinkOut - more resources
Full Text Sources

