Multiple control of azoquinoline based molecular photoswitches
- PMID: 38425524
- PMCID: PMC10901508
- DOI: 10.1039/d3sc05879d
Multiple control of azoquinoline based molecular photoswitches
Abstract
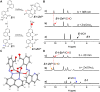
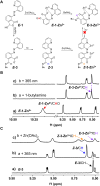
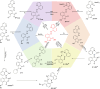
Multi-addressable molecular switches with high sophistication are creating intensive interest, but are challenging to control. Herein, we incorporated ring-chain dynamic covalent sites into azoquinoline scaffolds for the construction of multi-responsive and multi-state switching systems. The manipulation of ring-chain equilibrium by acid/base and dynamic covalent reactions with primary/secondary amines allowed the regulation of E/Z photoisomerization. Moreover, the carboxyl and quinoline motifs provided recognition handles for the chelation of metal ions and turning off photoswitching, with otherwise inaccessible Z-isomer complexes obtained via the change of stimulation sequence. Particularly, the distinct metal binding behaviors of primary amine and secondary amine products offered a facile way for modulating E/Z switching and dynamic covalent reactivity. As a result, multiple control of azoarene photoswitches was accomplished, including light, pH, metal ions, and amine nucleophiles, with interplay between diverse stimuli further enabling addressable multi-state switching within reaction networks. The underlying structural and mechanistic insights were elucidated, paving the way for the creation of complex switching systems, molecular assemblies, and intelligent materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Dynamic Covalent Switches and Communicating Networks for Tunable Multicolor Luminescent Systems and Vapor-Responsive Materials.J Am Chem Soc. 2019 Oct 16;141(41):16344-16353. doi: 10.1021/jacs.9b07175. Epub 2019 Oct 4. J Am Chem Soc. 2019. PMID: 31547653
-
Selection of isomerization pathways of multistep photoswitches by chalcogen bonding.Nat Commun. 2023 Nov 6;14(1):7139. doi: 10.1038/s41467-023-43013-8. Nat Commun. 2023. PMID: 37932318 Free PMC article.
-
Dual reactivity based dynamic covalent chemistry: mechanisms and applications.Chem Commun (Camb). 2023 Oct 31;59(87):12943-12958. doi: 10.1039/d3cc04022d. Chem Commun (Camb). 2023. PMID: 37772969 Review.
-
Photoswitchable dynamic conjugate addition-elimination reactions as a tool for light-mediated click and clip chemistry.Nat Commun. 2023 Jul 7;14(1):4015. doi: 10.1038/s41467-023-39669-x. Nat Commun. 2023. PMID: 37419874 Free PMC article.
-
Multiple Azoarenes Based Systems - Photoswitching, Supramolecular Chemistry and Application Prospects.Chem Rec. 2022 Nov;22(11):e202200074. doi: 10.1002/tcr.202200074. Epub 2022 Jul 21. Chem Rec. 2022. PMID: 35860915 Review.
Cited by
-
Three-state molecular switch based on terarylene photo- or redox-induced reversible isomerisation.Chem Sci. 2025 Jun 10;16(27):12499-12509. doi: 10.1039/d5sc02845k. eCollection 2025 Jul 10. Chem Sci. 2025. PMID: 40502824 Free PMC article.
-
Multiple pyrazolylazoindole/indazole scaffold based visible-light photoswitches with versatile controlled photophysical properties.Mol Divers. 2025 Mar 13. doi: 10.1007/s11030-025-11161-2. Online ahead of print. Mol Divers. 2025. PMID: 40080342
-
Multistate Dihydroazulene-Spiropyran Dyads: Path-Dependent Switchings and Refinement of the "Meta-rule" of Photoactivity.Chemistry. 2025 May 22;31(29):e202501061. doi: 10.1002/chem.202501061. Epub 2025 Apr 27. Chemistry. 2025. PMID: 40197595 Free PMC article.
-
Single-Wavelength Visible-Light-Induced Reversible Isomerization of Stiff-Stilbene under Dynamic Covalent Control.Org Lett. 2025 Apr 11;27(14):3612-3616. doi: 10.1021/acs.orglett.5c00707. Epub 2025 Mar 31. Org Lett. 2025. PMID: 40160032 Free PMC article.
References
-
- Chen H. Chen W. Lin Y. Xie Y. Liu S. H. Yin J. Visible and near-infrared light activated azo dyes. Chin. Chem. Lett. 2021;32:2359–2368. doi: 10.1016/j.cclet.2021.03.020. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

