Reconstruction of the human nipple-areolar complex: a tissue engineering approach
- PMID: 38425730
- PMCID: PMC10902434
- DOI: 10.3389/fbioe.2023.1295075
Reconstruction of the human nipple-areolar complex: a tissue engineering approach
Abstract
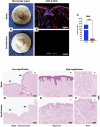
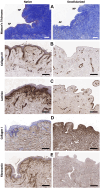
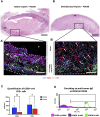
Introduction: Nipple-areolar complex (NAC) reconstruction after breast cancer surgery is challenging and does not always provide optimal long-term esthetic results. Therefore, generating a NAC using tissue engineering techniques, such as a decellularization-recellularization process, is an alternative option to recreate a specific 3D NAC morphological unit, which is then covered with an in vitro regenerated epidermis and, thereafter, skin-grafted on the reconstructed breast. Materials and methods: Human NACs were harvested from cadaveric donors and decellularized using sequential detergent baths. Cellular clearance and extracellular matrix (ECM) preservation were analyzed by histology, as well as by DNA, ECM proteins, growth factors, and residual sodium dodecyl sulfate (SDS) quantification. In vivo biocompatibility was evaluated 30 days after the subcutaneous implantation of native and decellularized human NACs in rats. In vitro scaffold cytocompatibility was assessed by static seeding of human fibroblasts on their hypodermal side for 7 days, while human keratinocytes were seeded on the scaffold epidermal side for 10 days by using the reconstructed human epidermis (RHE) technique to investigate the regeneration of a new epidermis. Results: The decellularized NAC showed a preserved 3D morphology and appeared white. After decellularization, a DNA reduction of 98.3% and the absence of nuclear and HLA staining in histological sections confirmed complete cellular clearance. The ECM architecture and main ECM proteins were preserved, associated with the detection and decrease in growth factors, while a very low amount of residual SDS was detected after decellularization. The decellularized scaffolds were in vivo biocompatible, fully revascularized, and did not induce the production of rat anti-human antibodies after 30 days of subcutaneous implantation. Scaffold in vitro cytocompatibility was confirmed by the increasing proliferation of seeded human fibroblasts during 7 days of culture, associated with a high number of living cells and a similar viability compared to the control cells after 7 days of static culture. Moreover, the RHE technique allowed us to recreate a keratinized pluristratified epithelium after 10 days of culture. Conclusion: Tissue engineering allowed us to create an acellular and biocompatible NAC with a preserved morphology, microarchitecture, and matrix proteins while maintaining their cell growth potential and ability to regenerate the skin epidermis. Thus, tissue engineering could provide a novel alternative to personalized and natural NAC reconstruction.
Keywords: ECM; decellularization; extracellular matrix; nipple–areolar complex; nipple–areolar complex reconstruction; recellularization; reconstructive surgery; tissue engineering.
Copyright © 2024 Maistriaux, Foulon, Fievé, Xhema, Evrard, Manon, Coyette, Bouzin, Poumay, Gianello, Behets and Lengelé.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Decellularized vascularized bone grafts: A preliminary in vitro porcine model for bioengineered transplantable bone shafts.Front Bioeng Biotechnol. 2023 Jan 18;10:1003861. doi: 10.3389/fbioe.2022.1003861. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36743653 Free PMC article.
-
Characterization of an Acellular Scaffold for a Tissue Engineering Approach to the Nipple-Areolar Complex Reconstruction.Cells Tissues Organs. 2017;203(3):183-193. doi: 10.1159/000455070. Epub 2017 Jan 27. Cells Tissues Organs. 2017. PMID: 28125805 Free PMC article.
-
An efficient strategy to recellularization of a rat aorta scaffold: an optimized decellularization, detergent removal, and Apelin-13 immobilization.Biomater Res. 2022 Sep 22;26(1):46. doi: 10.1186/s40824-022-00295-1. Biomater Res. 2022. PMID: 36138491 Free PMC article.
-
Lung Microvascular Niche, Repair, and Engineering.Front Bioeng Biotechnol. 2020 Feb 21;8:105. doi: 10.3389/fbioe.2020.00105. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32154234 Free PMC article. Review.
-
Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration.J Funct Biomater. 2023 Oct 16;14(10):518. doi: 10.3390/jfb14100518. J Funct Biomater. 2023. PMID: 37888183 Free PMC article. Review.
Cited by
-
Optimizing design parameters of 3D-printed poly-4-hydroxybutyrate nipple scaffolds for nipple reconstruction.Bioeng Transl Med. 2025 Apr 7;10(4):e70010. doi: 10.1002/btm2.70010. eCollection 2025 Jul. Bioeng Transl Med. 2025. PMID: 40708984 Free PMC article.
-
Technique for areolar reduction areolar-sparing mastectomy.J Surg Case Rep. 2024 Dec 27;2025(1):rjae824. doi: 10.1093/jscr/rjae824. eCollection 2025 Jan. J Surg Case Rep. 2024. PMID: 39737214 Free PMC article.
References
-
- American Society of Plastic Surgery (2020). Plastic surgery statistic report. Available at: https://www.plasticsurgery.org/documents/News/Statistics/2020/reconstruc... (Accessed January 3, 2023).
LinkOut - more resources
Full Text Sources
Research Materials

