Oncolytic alphavirus replicons mediated recruitment and activation of T cells
- PMID: 38425844
- PMCID: PMC10904282
- DOI: 10.1016/j.isci.2024.109253
Oncolytic alphavirus replicons mediated recruitment and activation of T cells
Abstract
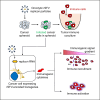
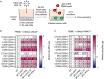
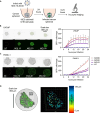
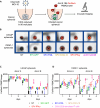
Oncolytic viruses show promise in enhancing tumor immunogenicity by releasing immunogenic signals during tumor cell infection and lysis. In this study, we improved the virus-induced tumor immunogenicity of recombinant Semliki Forest virus (rSFV)-based replicon particles by encoding immunogenic cytokines such as C-X-C motif chemokine ligand 10 (CXCL10), FMS-like tyrosine kinase 3 ligand (Flt3L), or interferon-gamma (IFN-ƴ). Real-time imaging and flow cytometry of human cancer cell-based monolayer and spheroid cultures, using LNCaP or PANC-1 cells, revealed effective infection and transgene expression in both models. LNCaP cells exhibited higher and earlier rSFV infection compared to PANC-1 cells. While infected LNCaP cells effectively triggered immune recruitment and T cell activation even without encoding cytokines, PANC-1 cells demonstrated improved immune responses only when infected with replicons encoding cytokines, particularly IFN-ƴ, which enhanced tumor immunogenicity irrespective of cancer cell susceptibility to infection. Our study demonstrates that despite innate phenotypic disparities in cancer cells, rSFV-based replicons encoding cytokines can potentially generate effective immune responses in the tumor.
Keywords: Immunology; Virology.
© 2024 The Author(s).
Conflict of interest statement
T.D. is co-founder of ViciniVax, a spin-off from the University of Groningen, developing therapeutic cancer vaccines.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

