Shape-Selective Supramolecular Capsules for Actinide Precipitation and Separation
- PMID: 38425904
- PMCID: PMC10900489
- DOI: 10.1021/jacsau.3c00793
Shape-Selective Supramolecular Capsules for Actinide Precipitation and Separation
Abstract
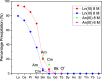
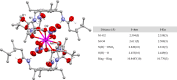
Improving actinide separations is key to reducing barriers to medical and industrial actinide isotope production and to addressing the challenges associated with the reprocessing of spent nuclear fuel. Here, we report the first example of a supramolecular anion recognition process that can achieve this goal. We have designed a preorganized triamidoarene receptor that induces quantitative precipitation of the early actinides Th(IV), Np(IV), and Pu(IV) from industrially relevant conditions through the formation of self-assembled hydrogen-bonded capsules. Selectivity over the later An(III) elements is shown through modulation of the nitric acid concentration, and no precipitation of actinyl or transition-metal ions occurs. The Np, Pu, and Am precipitates were characterized structurally by single-crystal X-ray diffraction and reveal shape specificity of the internal hydrogen-bonding array for the encapsulated hexanitratometalates. This work complements ion-exchange resins for 5f-element separations and illustrates the significant potential of supramolecular separation methods that target anionic actinide species.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Selective separation of light rare-earth elements by supramolecular encapsulation and precipitation.Nat Commun. 2022 Aug 3;13(1):4497. doi: 10.1038/s41467-022-32178-3. Nat Commun. 2022. PMID: 35922415 Free PMC article.
-
Complexation and redox chemistry of neptunium, plutonium and americium with a hydroxylaminato ligand.Chem Sci. 2021 Sep 3;12(40):13343-13359. doi: 10.1039/d1sc03905a. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777753 Free PMC article.
-
Advancing Understanding of the +4 Metal Extractant Thenoyltrifluoroacetonate (TTA-); Synthesis and Structure of MIVTTA4 (MIV = Zr, Hf, Ce, Th, U, Np, Pu) and MIII(TTA)4- (MIII = Ce, Nd, Sm, Yb).Inorg Chem. 2018 Apr 2;57(7):3782-3797. doi: 10.1021/acs.inorgchem.7b03089. Epub 2018 Mar 21. Inorg Chem. 2018. PMID: 29561140
-
Decisive Role of 5f-Orbital Covalence in the Structure and Stability of Pentavalent Transuranic Oxo [M6O8] Clusters.Inorg Chem. 2020 Dec 21;59(24):18068-18077. doi: 10.1021/acs.inorgchem.0c02539. Epub 2020 Dec 8. Inorg Chem. 2020. PMID: 33287539
-
Separation of actinides from spent nuclear fuel: A review.J Hazard Mater. 2016 Nov 15;318:266-281. doi: 10.1016/j.jhazmat.2016.07.027. Epub 2016 Jul 10. J Hazard Mater. 2016. PMID: 27427893 Review.
Cited by
-
Six degrees of actinide separation.Nat Rev Chem. 2024 Jun;8(6):408-409. doi: 10.1038/s41570-024-00610-5. Nat Rev Chem. 2024. PMID: 38698143 No abstract available.
References
-
- Mathur J. N.; Murali M. S.; Nash K. L. Actinide Partitioning—a Review. Solvent Extr. Ion Exch. 2001, 19 (3), 357–390. 10.1081/SEI-100103276. - DOI
-
- Robinson S. M.; Benker D. E.; Collins E. D.; Ezold J. G.; Garrison J. R.; Hogle S. L. Production of Cf-252 and Other Transplutonium Isotopes at Oak Ridge National Laboratory. Radiochim. Acta 2020, 108 (9), 737–746. 10.1515/ract-2020-0008. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
