Copper(II) Can Kinetically Trap Arctic and Italian Amyloid-β40 as Toxic Oligomers, Mimicking Cu(II) Binding to Wild-Type Amyloid-β42: Implications for Familial Alzheimer's Disease
- PMID: 38425915
- PMCID: PMC10900208
- DOI: 10.1021/jacsau.3c00687
Copper(II) Can Kinetically Trap Arctic and Italian Amyloid-β40 as Toxic Oligomers, Mimicking Cu(II) Binding to Wild-Type Amyloid-β42: Implications for Familial Alzheimer's Disease
Abstract
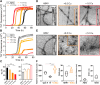
The self-association of amyloid-β (Aβ) peptide into neurotoxic oligomers is believed to be central to Alzheimer's disease (AD). Copper is known to impact Aβ assembly, while disrupted copper homeostasis impacts phenotype in Alzheimer's models. Here we show the presence of substoichiometric Cu(II) has very different impacts on the assembly of Aβ40 and Aβ42 isoforms. Globally fitting microscopic rate constants for fibril assembly indicates copper will accelerate fibril formation of Aβ40 by increasing primary nucleation, while seeding experiments confirm that elongation and secondary nucleation rates are unaffected by Cu(II). In marked contrast, Cu(II) traps Aβ42 as prefibrillar oligomers and curvilinear protofibrils. Remarkably, the Cu(II) addition to preformed Aβ42 fibrils causes the disassembly of fibrils back to protofibrils and oligomers. The very different behaviors of the two Aβ isoforms are centered around differences in their fibril structures, as highlighted by studies of C-terminally amidated Aβ42. Arctic and Italian familiar mutations also support a key role for fibril structure in the interplay of Cu(II) with Aβ40/42 isoforms. The Cu(II) dependent switch in behavior between nonpathogenic Aβ40 wild-type and Aβ40 Arctic or Italian mutants suggests heightened neurotoxicity may be linked to the impact of physiological Cu(II), which traps these familial mutants as oligomers and curvilinear protofibrils, which cause membrane permeability and Ca(II) cellular influx.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Naslund J.; Schierhorn A.; Hellman U.; Lannfelt L.; Roses A. D.; Tjernberg L. O.; Silberring J.; Gandy S. E.; Winblad B.; Greengard P.; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. U.S.A. 1994, 91 (18), 8378–8382. 10.1073/pnas.91.18.8378. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
