Generation and Reactivity of Polychalcogenide Chains in Binuclear Cobalt(II) Complexes
- PMID: 38425921
- PMCID: PMC10900221
- DOI: 10.1021/jacsau.3c00790
Generation and Reactivity of Polychalcogenide Chains in Binuclear Cobalt(II) Complexes
Abstract
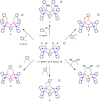
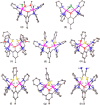
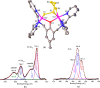
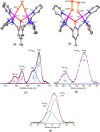
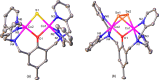
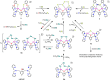
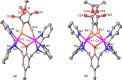
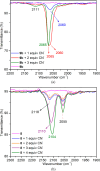
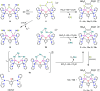
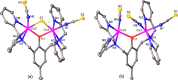
A series of six binuclear Co(II)-thiolate complexes, [Co2(BPMP)(S-C6H4-o-X)2]1+ (X = OMe, 2; NH2, 3), [Co2(BPMP)(μ-S-C6H4-o-O)]1+ (4), and [Co2(BPMP)(μ-Y)]1+ (Y = bdt, 5; tdt, 6; mnt, 7), has been synthesized from [Co2(BPMP)(MeOH)2(Cl)2]1+ (1a) and [Co2(BPMP)(Cl)2]1+ (1b), where BPMP1- is the anion of 2,6-bis[[bis(2-pyridylmethyl)amino]methyl]-4-methylphenol. While 2 and 3 could allow the two-electron redox reaction of the two coordinated thiolates with elemental sulfur (S8) to generate [Co2(BPMP)(μ-S5)]1+ (8), the complexes, 4-7, could not undergo a similar reaction. An analogous redox reaction of 2 with elemental selenium ([Se]) produced [{Co2(BPMP)(μ-Se4)}{Co2(BPMP)(μ-Se3)}]2+ (9a) and [Co2(BPMP)(μ-Se4)]1+ (9b). Further reaction of these polychalcogenido complexes, 8 and 9a/9b, with PPh3 allowed the isolation of [Co2(BPMP)(μ-S)]1+ (10) and [Co2(BPMP)(μ-Se2)]1+ (11), which, in turn, could be converted back to 8 and 9a upon treatment with S8 and [Se], respectively. Interestingly, while the redox reaction of the polyselenide chains in 9a and 11 with S8 produced 8 and [Se], the treatment of 8 with [Se] gave back only the starting material (8), thus demonstrating the different redox behavior of sulfur and selenium. Furthermore, the reaction of 8 and 9a/9b with activated alkynes and cyanide (CN-) allowed the isolation of the complexes, [Co2(BPMP)(μ-E2C2(CO2R)2)]1+ (E = S: 12a, R = Me; 12b, R = Et; E = Se: 13a, R = Me; 13b, R = Et) and [Co2(BPMP)(μ-SH)(NCS)2] (14), respectively. The present work, thus, provides an interesting synthetic strategy, interconversions, and detailed comparative reactivity of binuclear Co(II)-polychalcogenido complexes.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Thiolate Coordination vs C-S Bond Cleavage of Thiolates in Dinickel(II) Complexes.Inorg Chem. 2021 Jan 18;60(2):944-958. doi: 10.1021/acs.inorgchem.0c03068. Epub 2021 Jan 6. Inorg Chem. 2021. PMID: 33405907
-
Polysulfido Chain in Binuclear Zinc(II) Complexes.Inorg Chem. 2022 Apr 25;61(16):6295-6310. doi: 10.1021/acs.inorgchem.2c00555. Epub 2022 Apr 13. Inorg Chem. 2022. PMID: 35416644
-
Hydrolysis and Transfer Reactivity of the Coordinated Thiolate, Thiocarboxylate, and Selenolate in Binuclear Zinc(II) Complexes.Inorg Chem. 2023 Jul 17;62(28):11095-11111. doi: 10.1021/acs.inorgchem.3c01151. Epub 2023 Jul 6. Inorg Chem. 2023. PMID: 37409485
-
C-S Bond Cleavage, Redox Reactions, and Dioxygen Activation by Nonheme Dicobalt(II) Complexes.Inorg Chem. 2018 Jan 16;57(2):617-632. doi: 10.1021/acs.inorgchem.7b02432. Epub 2017 Dec 22. Inorg Chem. 2018. PMID: 29271646
-
Nonheme binuclear transition metal complexes with hydrosulfide and polychalcogenides.Chem Commun (Camb). 2024 May 7;60(38):4979-4998. doi: 10.1039/d4cc00929k. Chem Commun (Camb). 2024. PMID: 38654604 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
