Enantioselective Desymmetrization of Trifluoromethylated Tertiary Benzhydrols via Hydrogen-Acceptor-Free Ir-Catalyzed Dehydrogenative C-H Silylation: Decisive Role of the Trifluoromethyl Group
- PMID: 38425931
- PMCID: PMC10900501
- DOI: 10.1021/jacsau.3c00794
Enantioselective Desymmetrization of Trifluoromethylated Tertiary Benzhydrols via Hydrogen-Acceptor-Free Ir-Catalyzed Dehydrogenative C-H Silylation: Decisive Role of the Trifluoromethyl Group
Abstract
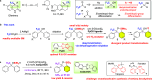
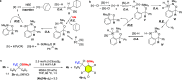
Although the trifluoromethyl (CF3) group is one of the most important fluorinated groups owing to its significant ability to modulate pharmacological properties, constructing trifluoromethylated stereogenic centers in an enantioselective manner has been a formidable challenge. Herein, we report the development of the enantioselective desymmetrization of trifluoromethylated benzhydrols via intramolecular dehydrogenative silylation using Ir catalysts with chiral pyridine-oxazoline (PyOX) ligands. The produced benzoxasilol was transformed into several unsymmetrical benzhydrols via iododesilylation and subsequent transition-metal-catalyzed cross-coupling reactions. Moreover, the same Ir catalyst system was used for the kinetic resolution of unsymmetrical trifluoromethylated benzhydrols.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. - DOI - PubMed
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. - DOI - PubMed
-
-
Selected reviews:
- Ma J.-A.; Cahard D. Strategies for nucleophilic, and radical trifluoromethylations. J. Fluorine Chem. 2007, 128, 975–996. 10.1016/j.jfluchem.2007.04.026. - DOI
- Liu H.; Gu Z.; Jiang X. Direct Trifluoromethylation of the C–H Bond. Adv. Synth. Catal. 2013, 355, 617–626. 10.1002/adsc.201200764. - DOI
- Zhu W.; Wang J.; Wang S.; Gu Z.; Aceña J. L.; Izawa K.; Liu H.; Soloshonok V. A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluorine Chem. 2014, 167, 37–54. 10.1016/j.jfluchem.2014.06.026. - DOI
- Barata-Vallejo S.; Postigo A. New Visible-Light-Triggered Photocatalytic Trifluoromethylation Reactions of Carbon–Carbon Multiple Bonds and (Hetero)Aromatic Compounds. Chem. - Eur. J. 2020, 26, 1065–11084. 10.1002/chem.202000856. - DOI - PubMed
- Aradi K.; Kiss L. Carbotrifluooromethylations of C–C Multiple Bonds (Excluding Aryl- and Alkynyltrifluoromethylations). Chem. - Eur. J. 2023, 29, e202203499 10.1002/chem.202203499. - DOI - PubMed
- Kawamura S.; Barrio P.; Fustero S.; Escorihuela J.; Han J.; Soloshonok V. A.; Sodeoka M. Evolution and Future of Hetero- and Hydro-Trifluoromethylations of Unsaturated C–C bonds. Adv. Synth. Catal. 2023, 365, 398–462. 10.1002/adsc.202201268. - DOI
-
-
- Li S.; Ma J.-A. Core-structure-inspired asymmetric addition reactions: enantioselective synthesis of dihydrobenzoxazinone- and dihydroquinazolinonne-based anti-HIV agents. Chem. Soc. Rev. 2015, 44, 7439–7448. 10.1039/C5CS00342C. - DOI - PubMed
- Bastos M. M.; Costa C. C. P.; Bezerra T. C.; de C da Silva F.; Boechat N. Efavirenz a nonnucleoside reverse transcriptase inhibitor of first-generation: Approaxhes based on its medicinal chemistry. Eur. J. Med. Chem. 2016, 108, 455–465. 10.1016/j.ejmech.2015.11.025. - DOI - PubMed
-
- Shishido Y.; Wakabayashi H.; Koike H.; Ueno N.; Nukui S.; Yamagishi T.; Murata Y.; Naganeo F.; Mizutani M.; Shimada K.; Fujiwara Y.; Sakakibara A.; Suga O.; Kusano R.; Ueda S.; Kanai Y.; Tsuchiya M.; Satake K. Discovery and stereoselective synthesis of the novel isochroman neurokinin-1 receptor antagonist ‘CJ-17,493′. Bioorg. Med. Chem. 2008, 16, 7193–7205. 10.1016/j.bmc.2008.06.047. - DOI - PubMed
-
- Ma J.-A.; Cahard D. Update 1 of: Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2008, 108, PR1–PR43. 10.1021/cr800221v. - DOI - PubMed
- Huang Y.-Y.; Yang X.; Chen Z.; Verpoort F.; Shibata N. Catalytic Asymmetric Synthesis of Enantioenriched Heterocycles Bearing a C–CF3 Stereogenic Center. Chem. - Eur. J. 2015, 21, 8664–8684. 10.1002/chem.201500361. - DOI - PubMed
- He X.-H.; Ji Y.-L.; Peng C.; Han B. Organocatalytic Asymmetric Synthesis of Cyclic Compounds Bearing a Trifluoromethylated Stereogenic Center: Recent Developments. Adv. Synth. Catal. 2019, 361, 1923–1957. 10.1002/adsc.201801647. - DOI
LinkOut - more resources
Full Text Sources
