Engineering Bacterial Biofilm Development and Structure via Regulation of Silver Nanoparticle Density in Graphene Oxide Composite Coating
- PMID: 38425932
- PMCID: PMC10900484
- DOI: 10.1021/jacsau.4c00008
Engineering Bacterial Biofilm Development and Structure via Regulation of Silver Nanoparticle Density in Graphene Oxide Composite Coating
Abstract
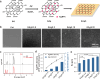
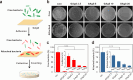
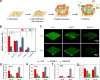
Graphene-based composites have shown significant potential in the treatment of biofilm infections in clinical settings due to their exceptional antimicrobial properties and specific mechanisms. Nevertheless, a comprehensive understanding of the influence exerted by nanoparticles embedded in the composites on the development and structure of biofilms is still lacking. Here, we fabricate different graphene oxide-silver nanoparticle (GAg) composite-modified substrates (GAgS) with varying densities of silver nanoparticles (AgNPs) and investigate their effects on planktonic bacterial adhesion, subsequent biofilm formation, and mature biofilm structure. Our findings indicate that the initial attachment of Pseudomonas aeruginosa cells during biofilm formation is determined by the density of AgNPs on the GAgS surface. In contrast, the subsequent transition from adherent bacteria to the biofilm is determined by GAgS's synergistic antimicrobial effect. There exists a threshold for the inhibitory performance of GAgS, where the 20 μg/cm2 GAg composite completely prevents biofilm formation; below this concentration, GAgS delays the development of the biofilm and causes structural changes in the mature biofilm with enhanced bacterial growth and increased production of extracellular polymeric substance. More importantly, GAgS have minimal impact on mammalian cell morphology and proliferation while not inducing hemolysis in red blood cells. These results suggest that GAg composites hold promise as a therapeutic approach for addressing medical devices and implant-associated biofilm infections.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
