Fisetin enhances cisplatin sensitivity in renal cell carcinoma via the CDK6/PI3K/Akt/mTOR signaling pathway
- PMID: 38426151
- PMCID: PMC10902757
- DOI: 10.3892/ol.2024.14298
Fisetin enhances cisplatin sensitivity in renal cell carcinoma via the CDK6/PI3K/Akt/mTOR signaling pathway
Abstract
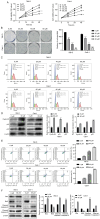
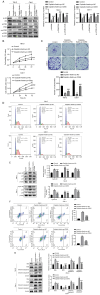
Cisplatin resistance is ubiquitous among patients with renal cell carcinoma (RCC). The present study assessed the role of fisetin in regulating cisplatin sensitivity and increasing the efficacy of chemotherapy for patients with RCC. Cell Counting Kit-8 and colony formation assays were used to assess the proliferation of RCC cells after fisetin and cisplatin treatment. The mRNA expression levels of cyclin-dependent kinase (CDK)6 were evaluated using reverse transcription-quantitative PCR. The expression levels of CDK6 and key proteins of the PI3K/Akt/mTOR signaling pathway were assessed using western blotting. The present study demonstrated that fisetin inhibited the proliferation and colony-forming ability of RCC cells, and induced apoptosis and cell cycle arrest in a dose-dependent manner. Additionally, fisetin enhanced the antineoplastic effects of cisplatin, as demonstrated by the increase in proliferation inhibition and apoptosis promotion after fisetin and cisplatin combination treatment. Furthermore, fisetin regulated the PI3K/Akt/mTOR signaling pathway through CDK6 inhibition, which enhanced cisplatin sensitivity. Overexpression of CDK6 neutralized the positive effects of fisetin on the improvement of cisplatin sensitivity in RCC cells. In conclusion, fisetin may enhance the sensitivity of RCC cells to cisplatin via the CDK6/PI3K/Akt/mTOR signaling pathway.
Keywords: cisplatin; drug resistance; fisetin; renal cell carcinoma.
Copyright: © Jiang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

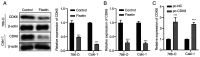
References
LinkOut - more resources
Full Text Sources
Miscellaneous
