Iodine‑125 seeds combined with carboplatin in the treatment of retroperitoneal metastatic seminoma: A case report and literature review
- PMID: 38426154
- PMCID: PMC10902756
- DOI: 10.3892/ol.2024.14289
Iodine‑125 seeds combined with carboplatin in the treatment of retroperitoneal metastatic seminoma: A case report and literature review
Abstract
Testicular seminoma is a relatively rare malignant tumor, with the most common site of recurrence and metastasis being the retroperitoneal lymph nodes. Since seminoma is highly sensitive to radiotherapy and chemotherapy, even if it metastasizes, its cure rate is still >95%. However, the long-term toxicity and side effects of radiotherapy and chemotherapy cannot be ignored. Iodine-125 seeds represent a low-energy radioactive source that kills tumor cells while protecting the surrounding normal tissues, and brachytherapy using iodine-125 seeds has been widely used for the treatment of various malignancies. In addition, carboplatin can be used as an alternative to cisplatin-based combination chemotherapy to reduce the incidence of pulmonary toxicity, neurological damage and renal toxicity. In the present study, a case in which iodine-125 seeds were implanted for the treatment of retroperitoneal metastatic seminoma is reported. The patient was diagnosed with postoperative recurrence of seminoma that metastasized to the retroperitoneal lymph nodes. Since the tumor was large and surrounded blood vessels, surgical intervention and external radiotherapy were not considered. Moreover, considering the potential long-term toxic side effects of standard chemotherapy, a treatment plan for the patient using iodine-125 seed implantation combined with carboplatin (AUC7) therapy was finally formulated. No disease recurrence or toxic reactions occurred during the 3-year follow-up after treatment. The present case therefore demonstrated the antitumor efficacy and reduced toxicity of iodine-125 seeds combined with carboplatin for treating seminoma.
Keywords: case report; iodine-125 seeds; retroperitoneal lymph node metastasis; seminoma.
Copyright: © Ran et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

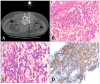



References
-
- Oldenburg J, Berney DM, Bokemeyer C, Climent MA, Daugaard G, Gietema JA, De Giorgi U, Haugnes HS, Huddart RA, Leão R, et al. Testicular seminoma and non-seminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:362–375. doi: 10.1016/j.annonc.2022.01.002. - DOI - PubMed
-
- Boormans JL, Sylvester R, Anson-Cartwright L, Glicksman RM, Hamilton RJ, Hahn E, Daugaard G, Lauritsen J, Wagner T, Avuzzi B, et al. Prognostic factor risk groups for clinical stage I seminoma: An individual patient data analysis by the European association of urology testicular cancer guidelines panel and guidelines office. Eur Urol Oncol. 2023 doi: 10.1016/j.euo.2023.10.014. S2588-9311(23)00232-8. (Epub ahead of print) - DOI - PubMed
-
- Giannatempo P, Greco T, Mariani L, Nicolai N, Tana S, Farè E, Raggi D, Piva L, Catanzaro M, Biasoni D, et al. Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: A systematic review and meta-analysis of patient outcomes. Ann Oncol. 2015;26:657–668. doi: 10.1093/annonc/mdu447. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
