Immune Regulatory Networks and Therapy of γδ T Cells in Liver Cancer: Recent Trends and Advancements
- PMID: 38426194
- PMCID: PMC10899867
- DOI: 10.14218/JCTH.2023.00355
Immune Regulatory Networks and Therapy of γδ T Cells in Liver Cancer: Recent Trends and Advancements
Abstract
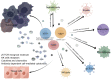
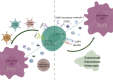
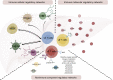
The roles of γδ T cells in liver cancer, especially in the potential function of immunotherapy due to their direct cytotoxic effects on tumor cells and secretion of important cytokines and chemokines, have aroused research interest. This review briefly describes the basic characteristics of γδ T cells, focusing on their diverse effects on liver cancer. In particular, different subtypes of γδ T cells have diverse or even opposite effects on liver cancer. We provide a detailed description of the immune regulatory network of γδ T cells in liver cancer from two aspects: immune components and nonimmune components. The interactions between various components in this immune regulatory network are dynamic and pluralistic, ultimately determining the biological effects of γδ T cells in liver cancer. We also integrate the current knowledge of γδ T-cell immunotherapy for liver cancer treatment, emphasizing the potential of these cells in liver cancer immunotherapy.
Keywords: Immunotherapy; Liver cancer; Tumor microenvironment; γδ T cells.
© 2024 Authors.
Conflict of interest statement
RL has been an editorial board member of Journal of Clinical and Translational Hepatology since 2022. The other authors have no conflict of interests related to this publication
Figures
Similar articles
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy.Signal Transduct Target Ther. 2023 Nov 22;8(1):434. doi: 10.1038/s41392-023-01653-8. Signal Transduct Target Ther. 2023. PMID: 37989744 Free PMC article. Review.
-
γδ T Cells for Leukemia Immunotherapy: New and Expanding Trends.Front Immunol. 2021 Sep 22;12:729085. doi: 10.3389/fimmu.2021.729085. eCollection 2021. Front Immunol. 2021. PMID: 34630403 Free PMC article. Review.
-
The functional impairment of HCC-infiltrating γδ T cells, partially mediated by regulatory T cells in a TGFβ- and IL-10-dependent manner.J Hepatol. 2013 May;58(5):977-83. doi: 10.1016/j.jhep.2012.12.015. Epub 2012 Dec 20. J Hepatol. 2013. PMID: 23262246
-
γδ T Cells and Tumor Microenvironment: From Immunosurveillance to Tumor Evasion.Front Immunol. 2018 Jun 15;9:1395. doi: 10.3389/fimmu.2018.01395. eCollection 2018. Front Immunol. 2018. PMID: 29963061 Free PMC article. Review.
-
γδ T cells: The potential role in liver disease and implications for cancer immunotherapy.J Leukoc Biol. 2022 Dec;112(6):1663-1668. doi: 10.1002/JLB.5MR0822-733RRR. Epub 2022 Sep 13. J Leukoc Biol. 2022. PMID: 36098208 Review.
Cited by
-
Liver immunology: Biological role and clinical significance.World J Hepatol. 2025 Jul 27;17(7):107541. doi: 10.4254/wjh.v17.i7.107541. World J Hepatol. 2025. PMID: 40747238 Free PMC article. Review.
-
Breast Cancer and Tumor Microenvironment: The Crucial Role of Immune Cells.Curr Oncol. 2025 Feb 28;32(3):143. doi: 10.3390/curroncol32030143. Curr Oncol. 2025. PMID: 40136347 Free PMC article. Review.
-
Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity.Int J Mol Sci. 2024 Aug 28;25(17):9331. doi: 10.3390/ijms25179331. Int J Mol Sci. 2024. PMID: 39273280 Free PMC article. Review.
-
Role of CD28+ PD-1+ Tc cells in immune response and prognosis prediction in hepatocellular carcinoma.Front Immunol. 2025 Jun 4;16:1576193. doi: 10.3389/fimmu.2025.1576193. eCollection 2025. Front Immunol. 2025. PMID: 40534863 Free PMC article.
-
The pancreatic tumor microenvironment of treatment-naïve patients causes a functional shift in γδ T cells, impairing their anti-tumoral defense.Oncoimmunology. 2025 Dec;14(1):2466301. doi: 10.1080/2162402X.2025.2466301. Epub 2025 Feb 13. Oncoimmunology. 2025. PMID: 39945298 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
