Volume loss during muscle reinnervation surgery is correlated with reduced CMAP amplitude but not reduced force output in a rat hindlimb model
- PMID: 38426207
- PMCID: PMC10902164
- DOI: 10.3389/fphys.2024.1328520
Volume loss during muscle reinnervation surgery is correlated with reduced CMAP amplitude but not reduced force output in a rat hindlimb model
Abstract
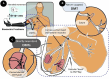
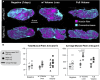
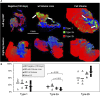
Introduction: Muscle reinnervation (MR) surgery offers rehabilitative benefits to amputees by taking severely damaged nerves and providing them with new denervated muscle targets (DMTs). However, the influence of physical changes to muscle tissue during MR surgery on long-term functional outcomes remains understudied. Methods: Our rat hindlimb model of MR surgery utilizes vascularized, directly neurotized DMTs made from the lateral gastrocnemius (LG), which we employed to assess the impact of muscle tissue size on reinnervation outcomes, specifically pairing the DMT with the transected peroneal nerve. We conducted MR surgery with both DMTs at full volume and DMTs with partial volume loss of 500 mg at the time of surgery (n = 6 per group) and measured functional outcomes after 100 days of reinnervation. Compound motor action potentials (CMAPs) and isometric tetanic force production was recorded from reinnervated DMTs and compared to contralateral naïve LG muscles as positive controls. Results: Reinnervated DMTs consistently exhibited lower mass than positive controls, while DMTs with partial volume loss showed no significant mass reduction compared to full volume DMTs (p = 0.872). CMAP amplitudes were lower on average in reinnervated DMTs, but a broad linear correlation also exists between muscle mass and maximum CMAP amplitude irrespective of surgical group (R2 = 0.495). Surprisingly, neither MR group, with or without volume loss, demonstrated decreased force compared to positive controls. The average force output of reinnervated DMTs, as a fraction of the contralateral LG's force output, approached 100% for both MR groups, a notable deviation from the 9.6% (±6.3%) force output observed in our negative control group at 7 days post-surgery. Tissue histology analysis revealed few significant differences except for a marked decrease in average muscle fiber area of reinnervated DMTs with volume loss compared to positive controls (p = 0.001). Discussion: The results from our rat model of MR suggests that tissue electrophysiology (CMAPs) and kinesiology (force production) may recover on different time scales, with volumetric muscle loss at the time of MR surgery not significantly reducing functional outcome measurements for the DMTs after 100 days of reinnervation.
Keywords: compound motor action potential amplitude; isometric tetanic force testing; muscle histology; muscle reinnervation; peripheral nerve.
Copyright © 2024 Lowe, Rivera Santana, Bopp, Quinn, Johnson, Ward, Chung, Tuffaha and Thakor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Motor units and histochemistry in rat lateral gastrocnemius and soleus muscles: evidence for dissociation of physiological and histochemical properties after reinnervation.J Neurophysiol. 1987 Apr;57(4):921-37. doi: 10.1152/jn.1987.57.4.921. J Neurophysiol. 1987. PMID: 2953872
-
Incomplete rematching of nerve and muscle properties in motor units after extensive nerve injuries in cat hindlimb muscle.J Physiol. 1998 Jun 15;509 ( Pt 3)(Pt 3):909-26. doi: 10.1111/j.1469-7793.1998.909bm.x. J Physiol. 1998. PMID: 9596809 Free PMC article.
-
Reinnervation of denervated muscle by implantation of nerve-muscle-endplate band graft to the native motor zone of the target muscle.Brain Behav. 2017 May 3;7(6):e00668. doi: 10.1002/brb3.668. eCollection 2017 Jun. Brain Behav. 2017. PMID: 28638701 Free PMC article.
-
Organization of motor units following cross-reinnervation of antagonistic muscles in the cat hind limb.J Physiol. 1986 May;374:443-56. doi: 10.1113/jphysiol.1986.sp016090. J Physiol. 1986. PMID: 3746699 Free PMC article.
-
Self-reinnervated cat medial gastrocnemius muscles. I. comparisons of the capacity for regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries.J Neurophysiol. 1996 Jan;75(1):268-81. doi: 10.1152/jn.1996.75.1.268. J Neurophysiol. 1996. PMID: 8822556
Cited by
-
NPD1 Relieves Neuropathic Pain and Accelerates the Recovery of Motor Function After Peripheral Nerve Injury.Pain Res Manag. 2024 Oct 30;2024:1109287. doi: 10.1155/2024/1109287. eCollection 2024. Pain Res Manag. 2024. PMID: 39512892 Free PMC article.
References
-
- Ahn S.-W., Yoon B.-N., Kim J.-E., Seok J. M., Kim K.-K., Kwon K.-H., et al. (2018). Nerve conduction studies: basic principal and clinical usefulness. Ann. Clin. Neurophysiology 20, 71–78. 10.14253/acn.2018.20.2.71 - DOI
LinkOut - more resources
Full Text Sources

