NEK2 plays an essential role in porcine embryonic development by maintaining mitotic division and DNA damage response via the Wnt/β-catenin signalling pathway
- PMID: 38426218
- PMCID: PMC11294417
- DOI: 10.1111/cpr.13626
NEK2 plays an essential role in porcine embryonic development by maintaining mitotic division and DNA damage response via the Wnt/β-catenin signalling pathway
Abstract
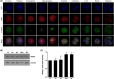
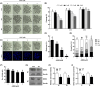
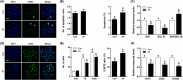
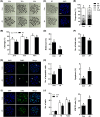
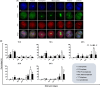
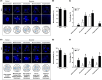
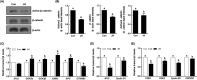
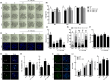
NIMA-related kinase 2 (NEK2) is a serine/threonine protein kinase that regulates mitosis and plays pivotal roles in cell cycle regulation and DNA damage repair. However, its function in porcine embryonic development is unknown. In this study, we used an NEK2-specific inhibitor, JH295 (JH), to investigate the role of NEK2 in embryonic development and the underlying regulatory mechanisms. Inhibition of NEK2 after parthenogenesis activation or in vitro fertilization significantly reduced the rates of cleavage and blastocyst formation, the numbers of trophectoderm and total cells and the cellular survival rate compared with the control condition. NEK2 inhibition delayed cell cycle progression at all stages from interphase to cytokinesis during the first mitotic division; it caused abnormal nuclear morphology in two- and four-cell stage embryos. Additionally, NEK2 inhibition significantly increased DNA damage and apoptosis, and it altered the expression levels of DNA damage repair- and apoptosis-related genes. Intriguingly, NEK2 inhibition downregulated the expression of β-catenin and its downstream target genes. To validate the relationship between Wnt/β-catenin signalling and NEK2 during porcine embryonic development, we cultured porcine embryos in JH-treated medium with or without CHIR99021, a Wnt activator. CHIR99021 co-treatment strongly restored the developmental parameters reduced by NEK2 inhibition to control levels. Our findings suggest that NEK2 plays an essential role in porcine embryonic development by regulating DNA damage repair and normal mitotic division via the Wnt/β-catenin signalling pathway.
© 2024 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

