Hypertension, Neurodegeneration, and Cognitive Decline
- PMID: 38426329
- PMCID: PMC11023809
- DOI: 10.1161/HYPERTENSIONAHA.123.21356
Hypertension, Neurodegeneration, and Cognitive Decline
Abstract
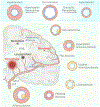
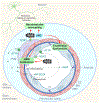
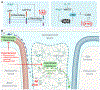
Elevated blood pressure is a well-established risk factor for age-related cognitive decline. Long linked to cognitive impairment on vascular bases, increasing evidence suggests a potential association of hypertension with the neurodegenerative pathology underlying Alzheimer disease. Hypertension is well known to disrupt the structural and functional integrity of the cerebral vasculature. However, the mechanisms by which these alterations lead to brain damage, enhance Alzheimer pathology, and promote cognitive impairment remain to be established. Furthermore, critical questions concerning whether lowering blood pressure by antihypertensive medications prevents cognitive impairment have not been answered. Recent developments in neurovascular biology, brain imaging, and epidemiology, as well as new clinical trials, have provided insights into these critical issues. In particular, clinical and basic findings on the link between neurovascular dysfunction and the pathobiology of neurodegeneration have shed new light on the overlap between vascular and Alzheimer pathology. In this review, we will examine the progress made in the relationship between hypertension and cognitive impairment and, after a critical evaluation of the evidence, attempt to identify remaining knowledge gaps and future research directions that may advance our understanding of one of the leading health challenges of our time.
Keywords: Alzheimer disease; blood pressure; brain; hypertension; stroke.
Conflict of interest statement
Figures
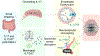
References
-
- Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–983. - PubMed
-
- Kety SS, Hafkenschiel JH. The blood flow, vascular resistance, and oxygen consumption of the brain in essential hypertension. The Journal of clinical investigation. 1948;27(4):511–514 - PubMed
-
- Leopold IH, Kety SS. Correlation of the cerebrovascular resistance and the grade of hypertensive retinal findings. American journal of ophthalmology. 1949;32(3):365–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

