A humanized IL-2 mutein expands Tregs and prolongs transplant survival in preclinical models
- PMID: 38426492
- PMCID: PMC10904054
- DOI: 10.1172/JCI173107
A humanized IL-2 mutein expands Tregs and prolongs transplant survival in preclinical models
Abstract
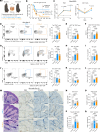
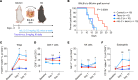
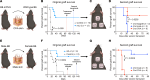
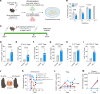
Long-term organ transplant survival remains suboptimal, and life-long immunosuppression predisposes transplant recipients to an increased risk of infection, malignancy, and kidney toxicity. Promoting the regulatory arm of the immune system by expanding Tregs may allow immunosuppression minimization and improve long-term graft outcomes. While low-dose IL-2 treatment can expand Tregs, it has a short half-life and off-target expansion of NK and effector T cells, limiting its clinical applicability. Here, we designed a humanized mutein IL-2 with high Treg selectivity and a prolonged half-life due to the fusion of an Fc domain, which we termed mIL-2. We showed selective and sustainable Treg expansion by mIL-2 in 2 murine models of skin transplantation. This expansion led to donor-specific tolerance through robust increases in polyclonal and antigen-specific Tregs, along with enhanced Treg-suppressive function. We also showed that Treg expansion by mIL-2 could overcome the failure of calcineurin inhibitors or costimulation blockade to prolong the survival of major-mismatched skin grafts. Validating its translational potential, mIL-2 induced a selective and sustainable in vivo Treg expansion in cynomolgus monkeys and showed selectivity for human Tregs in vitro and in a humanized mouse model. This work demonstrated that mIL-2 can enhance immune regulation and promote long-term allograft survival, potentially minimizing immunosuppression.
Keywords: Immunology; Organ transplantation; Tolerance; Transplantation.
Conflict of interest statement
Figures




Comment in
- Tipping the balance toward transplantation tolerance: in vivo therapy using a mutated IL-2
Similar articles
-
Notch-1 Inhibition Promotes Immune Regulation in Transplantation Via Regulatory T Cell-Dependent Mechanisms.Circulation. 2019 Sep 9;140(10):846-863. doi: 10.1161/CIRCULATIONAHA.119.040563. Epub 2019 Jul 3. Circulation. 2019. PMID: 31266349 Free PMC article.
-
IL-2 therapy preferentially expands adoptively transferred donor-specific Tregs improving skin allograft survival.Am J Transplant. 2019 Jul;19(7):2092-2100. doi: 10.1111/ajt.15306. Epub 2019 Mar 15. Am J Transplant. 2019. PMID: 30748096 Free PMC article.
-
Low dose interleukin-2 selectively expands circulating regulatory T cells but fails to promote liver allograft tolerance in humans.J Hepatol. 2023 Jan;78(1):153-164. doi: 10.1016/j.jhep.2022.08.035. Epub 2022 Sep 7. J Hepatol. 2023. PMID: 36087863 Clinical Trial.
-
The Next Frontier of Regulatory T Cells: Promising Immunotherapy for Autoimmune Diseases and Organ Transplantations.Front Immunol. 2020 Sep 23;11:565518. doi: 10.3389/fimmu.2020.565518. eCollection 2020. Front Immunol. 2020. PMID: 33072105 Free PMC article. Review.
-
Chimeric Antigen Receptor (CAR) Regulatory T-Cells in Solid Organ Transplantation.Front Immunol. 2022 May 26;13:874157. doi: 10.3389/fimmu.2022.874157. eCollection 2022. Front Immunol. 2022. PMID: 35720402 Free PMC article. Review.
Cited by
-
Belatacept and regulatory T cells in transplantation: synergistic strategies for immune tolerance and graft survival.Clin Transplant Res. 2024 Dec 31;38(4):326-340. doi: 10.4285/ctr.24.0057. Epub 2024 Dec 18. Clin Transplant Res. 2024. PMID: 39690903 Free PMC article. Review.
-
De-coupling immune parameters and toxicity associated with IL-12 agonism.Cell Rep. 2025 Jun 24;44(6):115840. doi: 10.1016/j.celrep.2025.115840. Epub 2025 Jun 12. Cell Rep. 2025. PMID: 40512622 Free PMC article.
-
Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells.Biomedicines. 2025 Jul 18;13(7):1757. doi: 10.3390/biomedicines13071757. Biomedicines. 2025. PMID: 40722827 Free PMC article. Review.
-
Inborn errors of regulatory T-cell differentiation and function.J Allergy Clin Immunol. 2025 Jul 7:S0091-6749(25)00741-9. doi: 10.1016/j.jaci.2025.07.001. Online ahead of print. J Allergy Clin Immunol. 2025. PMID: 40633593 Review.
-
Research Progress on Th17/Treg Cell Imbalance in Epileptic Seizures.J Inflamm Res. 2025 Jun 13;18:7769-7779. doi: 10.2147/JIR.S524814. eCollection 2025. J Inflamm Res. 2025. PMID: 40535356 Free PMC article. Review.

