PIEZO1 is a distal nephron mechanosensor and is required for flow-induced K+ secretion
- PMID: 38426496
- PMCID: PMC10904061
- DOI: 10.1172/JCI174806
PIEZO1 is a distal nephron mechanosensor and is required for flow-induced K+ secretion
Abstract
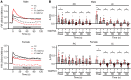
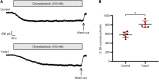
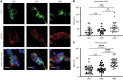
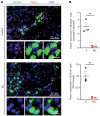
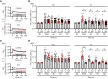
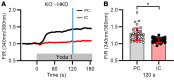
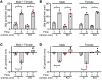
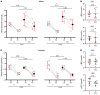
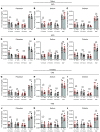
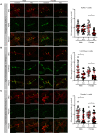
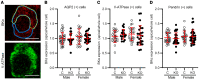
Ca2+-activated BK channels in renal intercalated cells (ICs) mediate luminal flow-induced K+ secretion (FIKS), but how ICs sense increased flow remains uncertain. We examined whether PIEZO1, a mechanosensitive Ca2+-permeable channel expressed in the basolateral membranes of ICs, is required for FIKS. In isolated cortical collecting ducts (CCDs), the mechanosensitive cation-selective channel inhibitor GsMTx4 dampened flow-induced increases in intracellular Ca2+ concentration ([Ca2+]i), whereas the PIEZO1 activator Yoda1 increased [Ca2+]i and BK channel activity. CCDs from mice fed a high-K+ (HK) diet exhibited a greater Yoda1-dependent increase in [Ca2+]i than CCDs from mice fed a control K+ diet. ICs in CCDs isolated from mice with a targeted gene deletion of Piezo1 in ICs (IC-Piezo1-KO) exhibited a blunted [Ca2+]i response to Yoda1 or increased flow, with an associated loss of FIKS in CCDs. Male IC-Piezo1-KO mice selectively exhibited an increased blood [K+] in response to an oral K+ bolus and blunted urinary K+ excretion following a volume challenge. Whole-cell expression of BKα subunit was reduced in ICs of IC-Piezo1-KO mice fed an HK diet. We conclude that PIEZO1 mediates flow-induced basolateral Ca2+ entry into ICs, is upregulated in the CCD in response to an HK diet, and is necessary for FIKS.
Keywords: Calcium channels; Cell biology; Epithelial transport of ions and water; Nephrology; Potassium channels.
Figures

Similar articles
-
Going with the flow: New insights regarding flow induced K+ secretion in the distal nephron.Physiol Rep. 2024 Oct;12(20):e70087. doi: 10.14814/phy2.70087. Physiol Rep. 2024. PMID: 39428258 Free PMC article. Review.
-
The mechanosensitive BKα/β1 channel localizes to cilia of principal cells in rabbit cortical collecting duct (CCD).Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F143-F156. doi: 10.1152/ajprenal.00256.2016. Epub 2016 Nov 2. Am J Physiol Renal Physiol. 2017. PMID: 27806944 Free PMC article.
-
Intercalated cell BKα subunit is required for flow-induced K+ secretion.JCI Insight. 2020 Apr 7;5(8):e130553. doi: 10.1172/jci.insight.130553. JCI Insight. 2020. PMID: 32255763 Free PMC article.
-
Role of NKCC in BK channel-mediated net K⁺ secretion in the CCD.Am J Physiol Renal Physiol. 2011 Nov;301(5):F1088-97. doi: 10.1152/ajprenal.00347.2011. Epub 2011 Aug 3. Am J Physiol Renal Physiol. 2011. PMID: 21816753 Free PMC article.
-
An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron.Am J Physiol Cell Physiol. 2016 Feb 15;310(4):C243-59. doi: 10.1152/ajpcell.00328.2015. Epub 2015 Dec 2. Am J Physiol Cell Physiol. 2016. PMID: 26632600 Free PMC article. Review.
Cited by
-
Paraoxonase 3 regulates the pore-forming α subunit of the large-conductance K+ channel.Biochim Biophys Acta Mol Cell Res. 2025 Aug;1872(6):119990. doi: 10.1016/j.bbamcr.2025.119990. Epub 2025 May 12. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 40368269 Free PMC article.
-
Mechanotransduction and the Integrity of the Slit Diaphragm.J Am Soc Nephrol. 2025 Mar 1;36(3):333-335. doi: 10.1681/ASN.0000000618. Epub 2025 Jan 28. J Am Soc Nephrol. 2025. PMID: 39874087 No abstract available.
-
Going with the flow: New insights regarding flow induced K+ secretion in the distal nephron.Physiol Rep. 2024 Oct;12(20):e70087. doi: 10.14814/phy2.70087. Physiol Rep. 2024. PMID: 39428258 Free PMC article. Review.
-
Aldosterone-independent regulation of K + secretion in the distal nephron.Curr Opin Nephrol Hypertens. 2024 Sep 1;33(5):526-534. doi: 10.1097/MNH.0000000000001006. Epub 2024 Jun 18. Curr Opin Nephrol Hypertens. 2024. PMID: 38888034 Review.
-
Hypertension research 2024 update and perspectives: basic research.Hypertens Res. 2024 Dec;47(12):3304-3309. doi: 10.1038/s41440-024-01878-2. Epub 2024 Sep 9. Hypertens Res. 2024. PMID: 39251854 Review.
References
-
- Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol. 1999;277(5):F805–F812. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

