SENP1 attenuates hypoxia‑reoxygenation injury in liver sinusoid endothelial cells by relying on the HIF‑1α signaling pathway
- PMID: 38426545
- PMCID: PMC10926105
- DOI: 10.3892/mmr.2024.13188
SENP1 attenuates hypoxia‑reoxygenation injury in liver sinusoid endothelial cells by relying on the HIF‑1α signaling pathway
Abstract
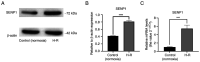
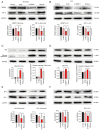
Liver sinusoidal endothelial cells (LSECs) have an important role in hepatic ischemia‑reperfusion injury (I/R), but the specific molecular mechanism of action is unknown. LSEC proliferation is regulated and fenestration is maintained via the Sentrin/SUMO‑specific protease 1 (SENP1)/hypoxia‑inducible factor‑1α (HIF‑1α) signaling axis under hypoxic conditions. In the present study, a hypoxia‑reoxygenation (H‑R) injury model was established using mouse LSECs to explore the relationship between SENP1 and H‑R injury in vitro, and the specific underlying mechanism was identified, revealing new targets for the clinical attenuation of hepatic I/R injury. Following the culture of LSECs under H‑R conditions, it was demonstrated that the expression of SENP1 was upregulated by reverse transcription‑quantitative polymerase chain reaction and western blotting (WB). In addition, scanning electron microscopy indicated that fenestrae damage was increased, a Cell Counting Kit‑8 assay demonstrated that the proliferation of cells was impaired and flow cytometry showed that apoptosis was increased. After silencing SENP1 expression with short interfering RNA, the proliferation activity of LSECs decreased, the fenestrae damage increased, the apoptosis rate increased and the expression levels of SENP1, HIF‑1α, heme oxygenase and Bcl‑2 were downregulated (as demonstrated by WB), while the expression levels of apoptosis‑related proteins, cleaved‑caspase‑3 and Bax, were upregulated. Enzyme‑linked immunosorbent assay detection showed that the level of vascular endothelial growth factor in the supernatant decreased and the level of IL‑6 and TNF‑α increased. Following the administration of an HIF‑1α signaling pathway agonist, the situation was reversed. These results therefore suggested that SENP1 attenuated the reduction in proliferation, apoptosis and fenestration of LSECs observed following H‑R injury through the HIF‑1α signaling pathway. In conclusion, SENP1 may attenuate H‑R injury in LSECs in a HIF‑1α signaling pathway‑dependent manner.
Keywords: Sentrin/SUMO- specific protease 1; hypoxia-reoxygenation injury; hypoxia‑inducible factor‑1α; liver sinusoidal endothelial cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


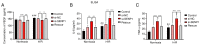
Similar articles
-
Hypoxia maintains the fenestration of liver sinusoidal endothelial cells and promotes their proliferation through the SENP1/HIF-1α/VEGF signaling axis.Biochem Biophys Res Commun. 2021 Feb 12;540:42-50. doi: 10.1016/j.bbrc.2020.12.104. Epub 2021 Jan 12. Biochem Biophys Res Commun. 2021. PMID: 33445109
-
Mechanism of SENP1-Mediated Regulation of Liver Sinusoidal Endothelial Cells to Promote Regeneration Via the HIF-1α Signaling Pathway.Arch Med Res. 2025 Aug 22;56(8):103295. doi: 10.1016/j.arcmed.2025.103295. Online ahead of print. Arch Med Res. 2025. PMID: 40848695
-
SENP‑1 enhances hypoxia‑induced proliferation of rat pulmonary artery smooth muscle cells by regulating hypoxia‑inducible factor‑1α.Mol Med Rep. 2016 Apr;13(4):3482-90. doi: 10.3892/mmr.2016.4969. Epub 2016 Mar 3. Mol Med Rep. 2016. PMID: 26935971 Free PMC article.
-
Liver Sinusoidal Endothelial Cell: An Update.Semin Liver Dis. 2017 Nov;37(4):377-387. doi: 10.1055/s-0037-1617455. Epub 2017 Dec 22. Semin Liver Dis. 2017. PMID: 29272898 Free PMC article. Review.
-
The vascular endothelial growth factor signaling pathway regulates liver sinusoidal endothelial cells during liver regeneration after partial hepatectomy.Expert Rev Gastroenterol Hepatol. 2021 Feb;15(2):139-147. doi: 10.1080/17474124.2020.1815532. Epub 2020 Oct 14. Expert Rev Gastroenterol Hepatol. 2021. PMID: 32902336 Review.
Cited by
-
Enteral nutrition intervention improves intestinal ischemia-reperfusion injury by modulating the SENP1/SIRT3 axis.J Clin Biochem Nutr. 2025 Jul;77(1):55-63. doi: 10.3164/jcbn.25-31. Epub 2025 Apr 9. J Clin Biochem Nutr. 2025. PMID: 40777823 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

