A Novel Dual-Fc Bispecific Antibody with Enhanced Fc Effector Function
- PMID: 38426700
- PMCID: PMC11025548
- DOI: 10.1021/acs.biochem.3c00481
A Novel Dual-Fc Bispecific Antibody with Enhanced Fc Effector Function
Abstract
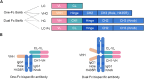
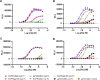
Bispecific antibodies (BsAbs) are undergoing continued development for applications in oncology and autoimmune diseases. While increasing activity by having more than one targeting arm, most BsAb engineering employs single Fc engagement as monoclonal antibodies. Here, we designed a novel immunoglobulin gamma-1 (IgG1)-derived dual-Fc BsAb containing two Fc regions and two distinct asymmetric antigen binding arms comprising a Fab arm and another VHH domain. In conjunction with the knob-into-hole technology, dual-Fc BsAbs could be produced with a high yield and good stability. We explore how Fc engineering effects on dual-Fc constructs could boost the desired therapeutic efficacy. This new format enabled simultaneous bispecific binding to corresponding antigens. Furthermore, compared to the one-Fc control molecules, dual-Fc BsAbs were shown to increase the avidity-based binding to FcγRs to result in higher ADCC and ADCP activities by potent avidity via binding to two antigens and Fc receptors. Overall, this novel BsAb format with enhanced effector functionalities provides a new option for antibody-based immunotherapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
A novel efficient bispecific antibody format, combining a conventional antigen-binding fragment with a single domain antibody, avoids potential heavy-light chain mis-pairing.J Immunol Methods. 2020 Aug;483:112811. doi: 10.1016/j.jim.2020.112811. Epub 2020 Jun 19. J Immunol Methods. 2020. PMID: 32569598
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
-
Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region.J Biochem. 2006 Sep;140(3):359-68. doi: 10.1093/jb/mvj157. Epub 2006 Jul 21. J Biochem. 2006. PMID: 16861252
-
Influence of the bispecific antibody IgG subclass on T cell redirection.MAbs. 2019 Aug/Sep;11(6):1012-1024. doi: 10.1080/19420862.2019.1624464. Epub 2019 Jun 26. MAbs. 2019. PMID: 31242061 Free PMC article.
-
Bispecific antibodies and trispecific immunocytokines for targeting the immune system against cancer: preparing for the future.BioDrugs. 2013 Feb;27(1):35-53. doi: 10.1007/s40259-012-0008-z. BioDrugs. 2013. PMID: 23329400 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

