Outcomes and device use in children with bone-conduction hearing devices in South Africa
- PMID: 38426736
- PMCID: PMC10913003
- DOI: 10.4102/sajcd.v71i1.1005
Outcomes and device use in children with bone-conduction hearing devices in South Africa
Abstract
Background: Bone-conduction hearing devices (BCHD) can provide hearing solutions in settings where middle ear pathology is rife.
Objectives: Describe functional hearing outcomes and device use of children fitted with BCHD.
Method: Retrospective review of 79 children fitted with BCHD between January 2017 and May 2022. Outcomes included device use and subjective reports measured with the Parents' Evaluation of Aural/Oral Performance of Children (PEACH) and the Teachers' Evaluation of Aural/Oral Performance of Children (TEACH). Analysis of variance established association between mean data logging and type and degree of hearing loss. Thematic analyses were done for qualitative outcomes.
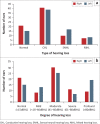
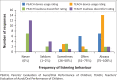
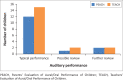
Results: Average usage was 7.0 h/day (5.4 SD; range 0.1-24). PEACH ratings indicated 93.3% of children wore their BCHD 'always' or 'often', with 80% displaying Typical auditory performance at 1-month follow-up. TEACH ratings indicated 84.2% of children wore their BCHD 'always' or 'often', with 78.9% showing typical auditory behaviour. Increased usage was noted for conductive, mixed, moderate and severe hearing losses. There was a mean delay of 17.2 months (23.4 SD; range 0-90) between age of diagnosis and fitting. Thematic analyses identified two main themes: advantages and barriers to BCDH use.
Conclusion: Average device use fell short of the internationally recommended 10 h/day. Higher BCHD use was associated with higher functional listening performance scores. Long waiting times for medical or surgical intervention for conductive hearing losses can delay BCHD fitting.Contribution: Limited information is available to examine outcomes in children fitted with BCHD.
Keywords: PEACH; TEACH.; bone conduction hearing devices; caregiver feedback; outcomes; paediatrics; teacher feedback.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

Similar articles
-
Outcomes of children with sensorineural hearing loss fitted with binaural hearing aids at a pediatric public hospital in South Africa.Int J Pediatr Otorhinolaryngol. 2022 Jan;152:110977. doi: 10.1016/j.ijporl.2021.110977. Epub 2021 Nov 13. Int J Pediatr Otorhinolaryngol. 2022. PMID: 34802750
-
Acquisition limitations of bone conduction hearing devices in children with unilateral microtia and atresia.Int J Pediatr Otorhinolaryngol. 2020 Jul;134:110040. doi: 10.1016/j.ijporl.2020.110040. Epub 2020 Apr 6. Int J Pediatr Otorhinolaryngol. 2020. PMID: 32361150
-
Bone conduction hearing device adherence in relationship to age in pediatric unilateral congenital aural atresia.Int J Pediatr Otorhinolaryngol. 2021 Feb;141:110564. doi: 10.1016/j.ijporl.2020.110564. Epub 2020 Dec 15. Int J Pediatr Otorhinolaryngol. 2021. PMID: 33340984
-
Clinical consensus document for fitting non-surgical transcutaneous bone conduction hearing devices to children.Int J Audiol. 2022 Jul;61(7):531-538. doi: 10.1080/14992027.2021.1939449. Epub 2021 Jul 13. Int J Audiol. 2022. PMID: 34255984 Review.
-
Non-implantable bone conduction device for hearing loss: a systematic review.J Biol Regul Homeost Agents. 2020 Sep-Oct;34(5 Suppl. 3):97-110. Technology in Medicine. J Biol Regul Homeost Agents. 2020. PMID: 33386039
References
-
- American Academy of Audiology . (2013). American academy of audiology: Clinical practice guidelines: Paediatric amplification. Retrieved from https://www.audiology.org/sites/default/files/publications/PaediatricAmp...
-
- Bagatto, M.P., Moodie, S.T., Seewald, R.C., Bartlett, D.J., & Scollie, S.D. (2011). A critical review of audiological outcome measures for infants and children. Trends in Amplification, 15(1), 23–33. https://doi.org/10.1177%2F1084713811412056 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
