A Dried Blood Spot protocol for high-throughput quantitative analysis of SARS-CoV-2 RBD serology based on the Roche Elecsys system
- PMID: 38426747
- PMCID: PMC10986497
- DOI: 10.1128/spectrum.02885-23
A Dried Blood Spot protocol for high-throughput quantitative analysis of SARS-CoV-2 RBD serology based on the Roche Elecsys system
Abstract
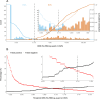
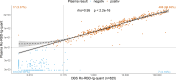
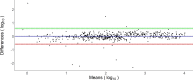
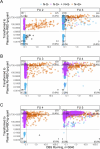
SARS-CoV-2 spreads pandemically since 2020; in 2021, effective vaccinations became available and vaccination campaigns commenced. Still, it is hard to track the spread of the infection or to assess vaccination success in the broader population. Measuring specific anti-SARS-CoV-2 antibodies is the most effective tool to track the spread of the infection or successful vaccinations. The need for venous-blood sampling however poses a significant barrier for large studies. Dried-blood-spots on filter-cards (DBS) have been used for SARS-CoV-2 serology in our laboratory, but so far not to follow quantitative SARS-CoV-2 anti-spike reactivity in a longitudinal cohort. We developed a semi-automated protocol or quantitative SARS-CoV-2 anti-spike serology from self-sampled DBS, validating it in a cohort of matched DBS and venous-blood samples (n = 825). We investigated chromatographic effects, reproducibility, and carry-over effects and calculated a positivity threshold as well as a conversion formula to determine the quantitative binding units in the DBS with confidence intervals. Sensitivity and specificity reached 96.63% and 97.81%, respectively, compared to the same test performed in paired venous samples. Between a signal of 0.018 and 250 U/mL, we calculated a correction formula. Measuring longitudinal samples during vaccinations, we demonstrated relative changes in titers over time in several individuals and in a longitudinal cohort over four follow-ups. DBS sampling has proven itself for anti-nucleocapsid serosurveys in our laboratory. Similarly, anti-spike high-throughput DBS serology is feasible as a complementary assay. Quantitative measurements are accurate enough to follow titer dynamics in populations also after vaccination campaigns. This work was supported by the Bavarian State Ministry of Science and the Arts; LMU University Hospital, LMU Munich; Helmholtz Center Munich; University of Bonn; University of Bielefeld; German Ministry for Education and Research (proj. nr.: 01KI20271 and others) and the Medical Biodefense Research Program of the Bundeswehr Medical Service. Roche Diagnostics provided kits and machines for analyses at discounted rates. The project is funded also by the European-wide Consortium ORCHESTRA. The ORCHESTRA project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 101016167. The views expressed in this publication are the sole responsibility of the author, and the Commission is not responsible for any use that may be made of the information it contains.IMPORTANCESARS-CoV-2 has been spreading globally as a pandemic since 2020. To determine the prevalence of SARS-CoV-2 antibodies among populations, the most effective public health tool is measuring specific anti-SARS-CoV-2 antibodies induced by infection or vaccination. However, conducting large-scale studies that involve venous-blood sampling is challenging due to the associated feasibility and cost issues. A more cost-efficient and less invasive method for SARS-CoV-2 serological testing is using Dried-Blood-Spots on filter cards (DBS). In this paper, we have developed a semi-automated protocol for quantifying SARS-CoV-2 anti-spike antibodies from self-collected DBS. Our laboratory has previously successfully used DBS sampling for anti-nucleocapsid antibody surveys. Likewise, conducting high-throughput DBS serology for anti-spike antibodies is feasible as an additional test that can be performed using the same sample preparation as the anti-nucleocapsid analysis. The quantitative measurements obtained are accurate enough to track the dynamics of antibody levels in populations, even after vaccination campaigns.
Keywords: COVID-19; DBS; Roche Elecsys; SARS-CoV-2; Spike; antibody; quantitative; serology.
Conflict of interest statement
The study was supported by multiple funders which includes Roche Diagnostics, providing machinery and kits for discounted rates. A.W. and M.H. have received personal (advisory) and material support from Roche, which was not related to the conduct of DBS serology presented within this study. The authors have no other relevant conflicts of interest to declare regarding the conduct of the study.
Figures


References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard 2023. https://covid19.who.int/.
-
- Centers for Disease Control and Prevention . C.D.T. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccine-effectiveness
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

