Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology
- PMID: 38426749
- PMCID: PMC11005406
- DOI: 10.1128/mbio.02623-23
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology
Abstract
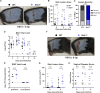
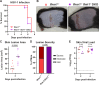
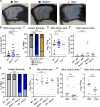
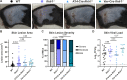
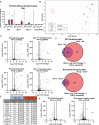
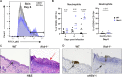
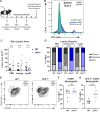
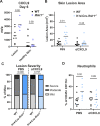
Type III interferons (IFN-λ) are antiviral and immunomodulatory cytokines that have been best characterized in respiratory and gastrointestinal infections, but the effects of IFN-λ against skin infections have not been extensively investigated. We sought to define the skin-specific effects of IFN-λ against the highly prevalent human pathogen, herpes simplex virus (HSV). We infected mice lacking the IFN-λ receptor (Ifnlr1-/-), both the IFN-λ and the IFN-α/β receptors (Ifnar1-/-Ifnlr1-/-), or IFN-λ cytokines (Ifnl2/3-/-) and found that IFN-λ restricts the severity of HSV-1 and HSV-2 skin lesions without affecting viral loads. We used RNAseq to define IFN-λ- and IFN-β-induced transcriptional responses in primary mouse keratinocytes. Using conditional knockout mice, we found that IFN-λ signaling in both keratinocytes and neutrophils was necessary to control HSV-1 skin lesion severity and that IFN-λ signaling in keratinocytes suppressed CXCL9-mediated neutrophil recruitment to the skin. Furthermore, depleting neutrophils or blocking CXCL9 protected against severe HSV-1 skin lesions in Ifnlr1-/- mice. Altogether, our results suggest that IFN-λ plays an immunomodulatory role in the skin that restricts neutrophil-mediated pathology during HSV infection and suggests potential applications for IFN-λ in treating viral skin infections.IMPORTANCEType III interferons (IFN-λ) have been shown to have antiviral and immunomodulatory effects at epithelial barriers such as the respiratory and gastrointestinal tracts, but their effects on the skin have not been extensively investigated. We used mice lacking IFN-λ signaling to investigate the skin-specific effects of IFN-λ against the herpes simplex virus (HSV), which targets epithelial tissues to cause cold sores and genital herpes. We found that IFN-λ limited the severity of HSV skin lesions without affecting viral load and that this protective effect required IFN-λ signaling in both keratinocytes and neutrophils. We found that IFN-λ signaling in keratinocytes suppressed neutrophil recruitment to the skin and that depleting neutrophils protected against severe HSV skin lesions in the absence of IFN-λ. Altogether, our results suggest that IFN-λ plays an immunomodulatory role in the skin that restricts neutrophil-mediated pathology during HSV infection and suggests potential applications for IFN-λ in treating viral skin infections.
Keywords: herpes simplex virus; interferon lambda; keratinocyte; neutrophils; skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology.bioRxiv [Preprint]. 2023 Sep 14:2023.09.11.557277. doi: 10.1101/2023.09.11.557277. bioRxiv. 2023. Update in: mBio. 2024 Apr 10;15(4):e0262323. doi: 10.1128/mbio.02623-23. PMID: 37745383 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

