Metabolic effect of adrenaline infusion in people with type 1 diabetes and healthy individuals
- PMID: 38427076
- PMCID: PMC11058679
- DOI: 10.1007/s00125-024-06116-5
Metabolic effect of adrenaline infusion in people with type 1 diabetes and healthy individuals
Abstract
Aims/hypothesis: As a result of early loss of the glucagon response, adrenaline is the primary counter-regulatory hormone in type 1 diabetes. Diminished adrenaline responses to hypoglycaemia due to counter-regulatory failure are common in type 1 diabetes, and are probably induced by exposure to recurrent hypoglycaemia, however, the metabolic effects of adrenaline have received less research attention, and also there is conflicting evidence regarding adrenaline sensitivity in type 1 diabetes. Thus, we aimed to investigate the metabolic response to adrenaline and explore whether it is modified by prior exposure to hypoglycaemia.
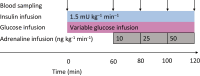
Methods: Eighteen participants with type 1 diabetes and nine healthy participants underwent a three-step ascending adrenaline infusion during a hyperinsulinaemic-euglycaemic clamp. Continuous glucose monitoring data obtained during the week before the study day were used to assess the extent of hypoglycaemia exposure.
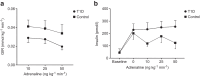
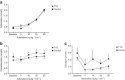
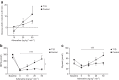
Results: While glucose responses during the clamp were similar between people with type 1 diabetes and healthy participants, plasma concentrations of NEFAs and glycerol only increased in the group with type 1 diabetes (p<0.001). Metabolomics revealed an increase in the most common NEFAs (p<0.01). Other metabolic responses were generally similar between participants with type 1 diabetes and healthy participants. Exposure to hypoglycaemia was negatively associated with the NEFA response; however, this was not statistically significant.
Conclusions/interpretation: In conclusion, individuals with type 1 diabetes respond with increased lipolysis to adrenaline compared with healthy participants by mobilising the abundant NEFAs in plasma, whereas other metabolic responses were similar. This may suggest that the metabolic sensitivity to adrenaline is altered in a pathway-specific manner in type 1 diabetes.
Trial registration: ClinicalTrials.gov NCT05095259.
Keywords: Clinical diabetes; Hypoglycaemia; Metabolic physiology in vivo; Metabolomics; Other hormones/action; Pathophysiology/metabolism.
© 2024. The Author(s).
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

