A Phase II Study of FOLFIRI Plus Ziv-Aflibercept After Trifluridine/Tipiracil Plus Bevacizumab in Patients with Metastatic Colorectal Cancer: WJOG 11018G
- PMID: 38427280
- PMCID: PMC10963434
- DOI: 10.1007/s11523-024-01043-2
A Phase II Study of FOLFIRI Plus Ziv-Aflibercept After Trifluridine/Tipiracil Plus Bevacizumab in Patients with Metastatic Colorectal Cancer: WJOG 11018G
Abstract
Background: Non-inferiority of trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) to irinotecan/fluoropyrimidine plus BEV in metastatic colorectal cancer was investigated in the phase III TRUSTY study, and we conducted a phase II study of FOLFIRI (5-FU+leucovorin+irinotecan) plus zib-aflibercept (AFL) after FTD/TPI plus BEV. However, the TRUSTY study failed during the recruitment of our patients.
Objective: We present the findings of a phase II study on the efficacy of FOLFIRI plus zib-aflibercept (AFL) after FTD/TPI plus BEV, including clinical results with plasma biomarker analyses.
Methods: This was a multicenter, single-arm, phase II study in patients with metastatic colorectal cancer refractory or intolerant to oxaliplatin, fluoropyrimidine, BEV, and FTD/TPI. The primary endpoint was progression-free survival. Fifteen plasma angiogenesis-associated biomarkers were analyzed using a Luminex® multiplex assay U-kit.
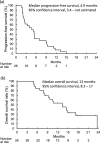
Results: Between January 2020 and May 2022, 26 patients (median age, 68 years) from 15 sites were enrolled. The median progression-free survival was 4.9 months (85% confidence interval, 3.4 month-not estimated). The overall response and disease control rates were 8% and 62%, respectively. The median levels of vascular endothelial growth factor-A and placental growth factor, both targets of AFL, were below the measurable limit of 30 pg/mL and 16 pg/mL, respectively. Patients were divided into two groups at the median levels of baseline biomarkers. The progression-free survival did not differ between high and low expressers of placental growth factor (p = 0.7), while it tended to be shorter in those with high levels of osteopontin (p = 0.05), angiopoietin-2 (p = 0.07), and tissue inhibitor of matrix metalloproteinases-1 (p = 0.1).
Conclusions: This study did not meet the primary endpoint. Hence, FOLFIRI plus AFL should not be used after FTD/TPI plus BEV for metastatic colorectal cancer. Further studies are needed to determine factors not targeted by AFL that may affect the efficacy of the treatment.
Clinical trial registration: jRCTs041190100.
© 2024. The Author(s).
Conflict of interest statement
Toshihiko Matsumoto received research funding from Ono Pharmaceutical Co., Ltd and Sanofi Co., Ltd; honoraria from Bayer Co., Ltd, Bristol-Myers Squibb Co., Ltd, Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Eli Lilly Japan Co., Ltd, Merck Bio Pharma Co., Ltd, MSD Co., Ltd, Ono Pharmaceutical Co., Ltd, Sanofi Co, Ltd, Taiho Pharmaceutical Co., Ltd, Takeda Co., Ltd, Teijin Pharmaceutical Co., Ltd, and Yakult Honsha Co., Ltd. Yoshiyuki Yamamoto received honoraria for lectures from Sanofi Co. Ltd, Ltd, Nihon Kayaku, Eisai, Bayer Co., Ltd, Lilly, Taiho Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Yakult Honsha Co., Ltd, Nihon Servier, Asahi Kasei, and Ono Pharmaceutical Co., Ltd. Masahito Kotaka has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai Pharmaceutical Co. Ltd, Taiho Pharmaceutical Co., Ltd, and Yakult Honsha Co., Ltd. Toshiki Masuishi has received institutional grants or contracts from MSD, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Novartis and personal consulting fees from Takeda Pharma, Chugai Pharmaceutical Co. Ltd, Merck Biopharma, Taiho Pharmaceutical Co., Ltd, Bayer, Eli Lilly Japan, Yakult Honsha Co., Ltd, Sanofi Co. Ltd, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Bristol Myers Squibb. Hirokazu Shoji has received honoraria from Ono Pharmaceutical Co., Ltd and Zymeworks Inc.; has been on advisory boards for Personal Amgen, Astellas, Daiichi Sankyo Co., Ltd, MSD, and Takeda Pharma; and a local PI. Akitaka Makiyama has received honoraria from Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co. Ltd., and Daiichi Sankyo Co. Ltd. Naoki Takahashi has received honoraria from Ono Pharmaceutical Co, Ltd, Taiho Pharmaceutical Co. Ltd, and Bristol Myers Squibb. Takashi Ohta has received honoraria from Eli Lilly, Bristol-Myers Squibb K.K., Taiho Pharmaceutical Co. Ltd, Ono Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd, and Merck & Co., Inc. Yosuke Kito has received honoraria from Taiho Pharmaceutical Co. Ltd, Ono Pharmaceutical Co., Ltd, and Daiichi Sankyo Co., Ltd. Narikazu Boku has received lecture fees or honoraria from Bristol-Myers Squibb, Daiichi-Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Taiho Pharmaceutical Co. Ltd, and research funds from Ono Pharmaceutical Co., Ltd and Takeda. Kentaro Yamazaki has received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Daiichi Sankyo Co., Ltd., Merck Biopharma Co., Ltd., Sanofi K.K., MSD K.K., Takeda Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Eli Lilly Japan K.K, Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb K.K. Shuichi Hironaka has received lecture fees or honoraria from Taiho Pharmaceutical Co., Ltd. Kei Muro has received lecture fees or honoraria from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Takeda Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd, and Taiho Pharmaceutical Co. Ltd; research funds from Chugai Pharmaceutical Co., Ltd., MSD K.K., Amgen K.K., Ono Pharmaceutical Co., Ltd., Astellas Pharma Inc., Sanofi Co., Ltd., Taiho Pharmaceutical Co. Ltd., Eisai Co., Ltd., Daiichi-Sankyo Co. Ltd., Novartis Pharma K.K., and Pfizer Japan Inc., and has been an advisor for AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., and Amgen K.K. Yasushi Tsuji, Kenro Hirata, Takao Tsuduki, Naoki Izawa, Masahiro Tsuda, Hisateru Yasui, Satoshi Otsu, Ichinosuke Hyodo, and Kenichi Yoshimura have no conflicts of interest that are directly relevant to the content of this article.
Figures

Similar articles
-
Exploratory Biomarker Analysis Using Plasma Angiogenesis-Related Factors and Cell-Free DNA in the TRUSTY Study: A Randomized, Phase II/III Study of Trifluridine/Tipiracil Plus Bevacizumab as Second-Line Treatment for Metastatic Colorectal Cancer.Target Oncol. 2024 Jan;19(1):59-69. doi: 10.1007/s11523-023-01027-8. Epub 2024 Jan 9. Target Oncol. 2024. PMID: 38194163 Free PMC article. Clinical Trial.
-
Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial.Br J Cancer. 2023 May;128(10):1897-1905. doi: 10.1038/s41416-023-02212-2. Epub 2023 Mar 4. Br J Cancer. 2023. PMID: 36871043 Free PMC article. Clinical Trial.
-
Trifluridine/Tipiracil Plus Bevacizumab for Vulnerable Patients With Pretreated Metastatic Colorectal Cancer: A Retrospective Study (WJOG14520G).Oncologist. 2024 Mar 4;29(3):e330-e336. doi: 10.1093/oncolo/oyad296. Oncologist. 2024. PMID: 37950903 Free PMC article.
-
Efficacy and Safety of Trifluridine/Tipiracil-Containing Combinations in Colorectal Cancer and Other Advanced Solid Tumors: A Systematic Review.Oncologist. 2024 May 3;29(5):e601-e615. doi: 10.1093/oncolo/oyae007. Oncologist. 2024. PMID: 38366864 Free PMC article.
-
Clinical Trial Data Review of the Combination FTD/TPI + Bevacizumab in the Treatment Landscape of Unresectable Metastatic Colorectal Cancer.Curr Treat Options Oncol. 2024 Oct;25(10):1312-1322. doi: 10.1007/s11864-024-01261-w. Epub 2024 Sep 26. Curr Treat Options Oncol. 2024. PMID: 39325367 Free PMC article. Review.
References
-
- Chiron M, Bagley RG, Pollard J, Henry C, Mankoo LV, Vincent L, et al. Switching to aflibercept treatment resulted in greater tumor responses than continuous bevacizumab treatment in patient-derived xenograft models of colorectal cancer. In: Proceedings of the 2013 AACR-NCI-EORTC International Conference on Molecular Targets. Mol Cancer Ther. 2013;12:11:Abstract B2.
-
- Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarrulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. - DOI - PubMed
-
- Sims TN, Gao B, Chiron M, Mancini P, Bagley R, Lowy I. Potential predictive and prognostic biomarkers identified in baseline plasma samples from the VELOUR trial. J Clin Oncol. 2015;33(3_Suppl.638).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

