Comparative efficacy and safety of stiripentol, cannabidiol and fenfluramine as first-line add-on therapies for seizures in Dravet syndrome: A network meta-analysis
- PMID: 38427284
- PMCID: PMC10984299
- DOI: 10.1002/epi4.12923
Comparative efficacy and safety of stiripentol, cannabidiol and fenfluramine as first-line add-on therapies for seizures in Dravet syndrome: A network meta-analysis
Abstract
Objectives: Stiripentol, fenfluramine, and cannabidiol are licensed add-on therapies to treat seizures in Dravet Syndrome (DS). There are no direct or indirect comparisons assessing their full licensed dose regimens, across different jurisdictions, as first-line add-on therapies in DS.
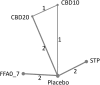
Methods: We conducted a systematic review and frequentist network meta-analysis (NMA) of randomized controlled trial (RCT) data for licensed add-on DS therapies. We compared the proportions of patients experiencing: reductions from baseline in monthly convulsive seizure frequency (MCSF) of ≥50% (clinically meaningful), ≥75% (profound), and 100% (seizure-free); serious adverse events (SAEs); discontinuations due to AEs.
Results: We identified relevant data from two placebo-controlled RCTs for each drug. Stiripentol 50 mg/kg/day and fenfluramine 0.7 mg/kg/day had similar efficacy in achieving ≥50% (clinically meaningful) and ≥75% (profound) reductions from baseline in MCSF (absolute risk difference [RD] for stiripentol versus fenfluramine 1% [95% confidence interval: -20% to 22%; p = 0.93] and 6% [-15% to 27%; p = 0.59], respectively), and both were statistically superior (p < 0.05) to licensed dose regimens of cannabidiol (10 or 20 mg/kg/day, with/irrespective of clobazam) for these outcomes. Stiripentol was statistically superior in achieving seizure-free intervals compared to fenfluramine (RD = 26% [CI: 8% to 44%; p < 0.01]) and licensed dose regimens of cannabidiol. There were no significant differences in the proportions of patients experiencing SAEs. The risk of discontinuations due to AEs was lower for stiripentol, although the stiripentol trials were shorter.
Significance: This NMA of RCT data indicates stiripentol, as a first-line add-on therapy in DS, is at least as effective as fenfluramine and both are more effective than cannabidiol in reducing convulsive seizures. No significant difference in the incidence of SAEs between the three add-on agents was observed, but stiripentol may have a lower risk of discontinuations due to AEs. These results may inform clinical decision-making and the continued development of guidelines for the treatment of people with DS.
Plain language summary: This study compared three drugs (stiripentol, fenfluramine, and cannabidiol) used alongside other medications for managing seizures in a severe type of epilepsy called DS. The study found that stiripentol and fenfluramine were similarly effective in reducing seizures and both were more effective than cannabidiol. Stiripentol was the best drug for stopping seizures completely based on the available clinical trial data. All three drugs had similar rates of serious side effects, but stiripentol had a lower chance of being stopped due to side effects. This information can help guide treatment choices for people with DS.
Keywords: Dravet syndrome; cannabidiol; fenfluramine; network meta‐analysis; stiripentol.
© 2024 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
RG received fees for Advisory Boards from UCB, Zogenix, Biocodex, GW‐Jazz, Angelini, Takeda, and Rapport Therapeutics, and was an investigator in the STICLO‐Italy trial of stiripentol. CC received fees for Advisory Boards from Advicenne, Zogenix, Neuren, Biocodex, EISAI, GW‐Jazz, BIAL, and Orphelia, and was an investigator in the STICLO‐France trial of stiripentol. DV is an employee of Biocodex, the manufacturer of stiripentol. TT is a past employee of Zogenix International Ltd/UCB, is Director of Henley Health Economics Ltd and received consulting fees from Biocodex for this and other projects. WL is a past employee of Zogenix International Ltd/UCB, is Director of Paragon Market Access Ltd and received consulting fees from Henley Health Economics Ltd for this and other projects. All authors abided by their ongoing confidentiality and contractual obligations.
Figures


References
-
- Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. - PubMed
-
- Connolly M. Dravet syndrome: diagnosis and long‐term course. Can J Neurol Sci. 2016;43:S3–S8. - PubMed
-
- Nabbout R, Auvin S, Chiron C, Irwin J, Mistry A, Bonner N, et al. Development and content validation of a preliminary core set of patient‐ and caregiver‐relevant outcomes for inclusion in a potential composite endpoint for Dravet syndrome. Epilepsy Behav. 2018;78:232–242. - PubMed
-
- Knupp KG, Scarbro S, Wilkening G, Juarez‐Colunga E, Kempe A, Dempsey A. Parental perception of comorbidities in children with Dravet syndrome. Pediatr Neurol. 2017;76:60–65. - PubMed
-
- Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

