Dalbavancin Sequential Therapy for Gram-Positive Bloodstream Infection: A Multicenter Observational Study
- PMID: 38427289
- PMCID: PMC10965835
- DOI: 10.1007/s40121-024-00933-2
Dalbavancin Sequential Therapy for Gram-Positive Bloodstream Infection: A Multicenter Observational Study
Abstract
Introduction: Long-acting lipoglycopeptides such as dalbavancin may have utility in patients with Gram-positive bloodstream infections (BSI), particularly in those with barriers to discharge or who require prolonged parenteral antibiotic courses. A retrospective cohort study was performed to provide further multicenter real-world evidence on dalbavancin use as a sequential therapy for Gram-positive BSI.
Methods: One hundred fifteen patients received dalbavancin with Gram-positive BSI, defined as any positive blood culture or diagnosed with infective endocarditis, from 13 centers geographically spread across the United States between July 2015 and July 2021.
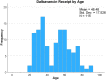
Results: Patients had a mean (SD) age of 48.5 (17.5) years, the majority were male (54%), with many who injected drugs (40%). The most common infection sources (non-exclusive) were primary BSI (89%), skin and soft tissue infection (SSTI) (25%), infective endocarditis (19%), and bone and joint infection (17%). Staphylococcus aureus accounted for 72% of index cultures, coagulase-negative Staphylococcus accounted for 18%, and Streptococcus species in 16%. Dalbavancin started a median (Q1-Q3) of 10 (6-19) days after index culture collection. The most common regimen administered was dalbavancin 1500 mg as one dose for 50% of cases. The primary outcome of composite clinical failure occurred at 12.2%, with 90-day mortality at 7.0% and 90-day BSI recurrence at 3.5%.
Conclusions: Dalbavancin may serve as a useful tool in facilitating hospital discharge in patients with Gram-positive BSI. Randomized controlled trials are anticipated to validate dalbavancin as a surrogate to current treatment standards.
Keywords: Staphylococcus; Streptococcus; Bacteremia; Bloodstream infection; Dalbavancin; Glycopeptides; Injection drug use.
© 2024. The Author(s).
Conflict of interest statement
Nicholas Rebold, Jeffery C. Pearson, Brandon Dionne, Ahmad Taqi, Abdalhamid Lagnf, Kristen Lucas, Mark Biagi, Nicholas Lombardo, Joshua Eudy, Daniel T. Anderson, Joseph A. D’Antonio, Jeremy J Frens, Tyler Baumeister, Matthew Geriak, Dino Delaportas, Jeremy Larew, and Michael P. Veve have no conflicts of interest to report. Sara Alosaimy is a current employee of Seres Therapeutics. Monica V. Mahoney has received funds for research from Merck; consulting funds from Cidara, Melinta, Merck, Pfizer, Qpex, and Spero; speaker honoraria from Paratek. Wesley D. Kufel has received research grants from Merck and Melinta; served on the advisory board for Theratechnologies, Inc.; Bruce M. Jones has received funds for consulting or participated in speaking bureaus for AbbVie, Melinta, Merck, Paratek, and La Jolla. George Sakoulas has consulted for Octapharma, AbbVie, and Paratek Pharmaceuticals and is on the speakers bureau for AbbVie and Paratek Pharmaceuticals. Dimitrios Farmakiotis has received research support from Astellas, Viracor and Merck, and consultation fee from Viracor. Michael J. Rybak has received funds for research and consulting or participated in speaking bureaus for AbbVie, Ferring, Melinta, Merck, Paratek Pharmaceuticals, Shionogi, Spero, La Jolla, and T2 Bioscience and was/is partially supported by National Institute of Allergy and Infectious Diseases R01 AI121400 and R21 AI163726. Michael J. Rybak is Editor in Chief of Infectious Diseases and Therapy. Michael J. Rybak was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
-
- Cooper GL, Given DB. Vancomycin: a comprehensive review of 30 years of clinical experience. Park Row Publishing Incorporated A Wiley and Sons Medical Group Comp; 1986.
LinkOut - more resources
Full Text Sources