HIV Preexposure Prophylaxis With Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women
- PMID: 38427359
- PMCID: PMC10951736
- DOI: 10.1001/jama.2024.0464
HIV Preexposure Prophylaxis With Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women
Abstract
Importance: Emtricitabine and tenofovir disoproxil fumarate (F/TDF) for HIV preexposure prophylaxis (PrEP) is highly effective in cisgender men who have sex with men (MSM) when adherence is high (>4 doses/week). Real-world effectiveness and adherence with F/TDF for PrEP in cisgender women is less well characterized.
Objective: To characterize the effectiveness of F/TDF for PrEP and its relationship with adherence in cisgender women.
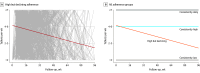
Design, setting, and participants: Data were pooled from 11 F/TDF PrEP postapproval studies conducted in 6 countries that included 6296 cisgender women aged 15 to 69 years conducted from 2012 to 2020. HIV incidence was evaluated according to adherence level measured objectively (tenofovir diphosphate concentration in dried blood spots or tenofovir concentration in plasma; n = 288) and subjectively (electronic pill cap monitoring, pill counts, self-report, and study-reported adherence scale; n = 2954) using group-based trajectory modeling.
Exposures: F/TDF prescribed orally once a day. HIV incidence was analyzed in subgroups based on adherence trajectory.
Main outcomes and measures: HIV incidence.
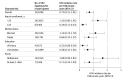
Results: Of the 6296 participants, 46% were from Kenya, 28% were from South Africa, 21% were from India, 2.9% were from Uganda, 1.6% were from Botswana, and 0.8% were from the US. The mean (SD) age at PrEP initiation across all studies was 25 (7) years, with 61% of participants being younger than 25 years. The overall HIV incidence was 0.72 per 100 person-years (95% CI, 0.51-1.01; 32 incident HIV diagnoses among 6296 participants). Four distinct groups of adherence trajectories were identified: consistently daily (7 doses/week), consistently high (4-6 doses/week), high but declining (from a mean of 4-6 doses/week and then declining), and consistently low (less than 2 doses/week). None of the 498 women with consistently daily adherence acquired HIV. Only 1 of the 658 women with consistently high adherence acquired HIV (incidence rate, 0.13/100 person-years [95% CI, 0.02-0.92]). The incidence rate was 0.49 per 100 person-years (95% CI, 0.22-1.08) in the high but declining adherence group (n = 1166) and 1.27 per 100 person-years (95% CI, 0.53-3.04) in the consistently low adherence group (n = 632).
Conclusions and relevance: In a pooled analysis of 11 postapproval studies of F/TDF for PrEP among cisgender women, overall HIV incidence was 0.72 per 100 person-years; individuals with consistently daily or consistently high adherence (4-6 doses/week) to PrEP experienced very low HIV incidence.
Conflict of interest statement
Figures


Comment in
-
Shifting the Narrative of Preexposure Prophylaxis Adherence Counseling for Cisgender Women.JAMA. 2024 Mar 19;331(11):912-914. doi: 10.1001/jama.2024.0432. JAMA. 2024. PMID: 38427361 No abstract available.
References
-
- World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach: 2021. update. Updated July 16, 2021. Accessed October 10, 2022. https://apps.who.int/iris/handle/10665/342899 - PubMed
-
- US Food and Drug Administration . FDA approves first drug for reducing the risk of sexually acquired HIV infection. Published July 16, 2012. Accessed April 3, 2023. https://www.hiv.gov/blog/fda-approves-first-drug-for-reducing-the-risk-o...
-
- National Institute of Child Health and Human Development . Item of interest: FDA approves PrEP therapy for adolescents at risk of HIV. Published May 16, 2018. Accessed April 11, 2023. https://www.nichd.nih.gov/newsroom/news/051618-PrEP
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

