Safety Profile of Pimavanserin Therapy in Elderly Patients with Neurodegenerative Disease-Related Neuropsychiatric Symptoms: A Phase 3B Study
- PMID: 38427485
- PMCID: PMC10977351
- DOI: 10.3233/JAD-231167
Safety Profile of Pimavanserin Therapy in Elderly Patients with Neurodegenerative Disease-Related Neuropsychiatric Symptoms: A Phase 3B Study
Abstract
Background: Pimavanserin, a 5-HT2A receptor inverse agonist/antagonist, is the only medication approved by the FDA for the treatment of hallucinations and delusions associated with Parkinson's disease psychosis (PDP). Further expanding knowledge of the safety profile of pimavanserin in PDP and neurodegenerative diseases (NDD) such as Alzheimer's disease is of great interest for informing its use in patients with PDP (with or without dementia), given this population is highly sensitive to adverse effects following antipsychotic use.
Objective: This trial evaluated the effects of pimavanserin compared to placebo in frail older adults and elderly patients with neuropsychiatric symptoms related to NDD, such as hallucinations and delusions, to better understand the safety of pimavanserin in this population.
Methods: This was a phase 3b, 8-week treatment (study duration of up to 16 weeks), multicenter, randomized, double-blind, placebo-controlled, two-arm parallel-group trial (NCT03575052). The primary endpoint was safety and tolerability, measured by treatment-emergent adverse events (TEAEs). Secondary safety endpoints were change from baseline in motor and cognitive function; exploratory endpoints included suicidality, sleep quality, and neuropsychiatric symptoms.
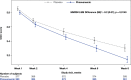
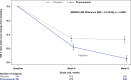
Results: Incidences of TEAEs were similar between treatment groups; 29.8% reported ≥1 TEAE (pimavanserin: 30.4%; placebo: 29.3%), and 1.8% reported serious TEAEs (pimavanserin: 2.0%; placebo: 1.5%). Pimavanserin did not impact motor- or cognitive-related function.
Conclusions: Pimavanserin was well tolerated and not associated with motor or cognitive impairment. Together, these findings highlight the manageable and generally favorable safety profile of pimavanserin in patients with NDD, contributing to our knowledge on the safety of pimavanserin as it generalizes to patients with PDP.
Keywords: Alzheimer’s disease; elderly patients; neurodegenerative diseases; neuropsychiatric symptoms; pimavanserin; safety.
Conflict of interest statement
GA: Research support from Accera, Allergan, Axovant, Eisai, Neurotrope, Genentech, Intra Cellular, Janssen, Lundbeck, Neurim, Novartis, Otsuka, Roche, Suven, and Trans Tech. Speakers Bureau and Consultant for Acadia, Alkermes, Allergan, Avanir, Janssen, Lundbeck, Merck, Nestle, Otsuka, Sunovion, Takeda, and Vanda. WJC: Received grants from Acadia, Alkermes, Allergan, Angelini, Auspex Pharmaceuticals, BMS, Celon, Cephalon, Cortexyme, Ferrier, Forest Laboratories, GedeonRichter, GW Pharmaceuticals, HMNC Brain Health, IntraCellular Therapies, Janssen, KCR, Lilly, Lundbeck, Minerva, MSD, NIH, Novartis, Orion, Otsuka, Sanofi, and Servier; received honoraria from Adamed, Angelini, AstraZeneca, Bristol Myers Squibb, Celon, GSK, Janssen, KRKA, Lekam, Lundbeck, Minerva, NeuroCog, Novartis, Orion, Pfizer, Polfa Tarchomin, Sanofi, Servier, and Zentiva; served on advisory boards for Angelini, Celon (terminated), Douglas Pharmaceuticals, Janssen, MSD, Novartis, and Sanofi.
VA, AB, SP, and BC: employees of ACADIA Pharmaceuticals Inc. (San Diego, CA, USA) at the time of the study.
Figures

References
-
- FDA, FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease, https://www.fda.gov/news-events/press-announcements/fda-approves-first-d..., Accessed June 14.
-
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf BR, Brann MR, Davis RE (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phen ylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist, J Pharmacol Exp Ther 317, 910–918. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

