Disease Trajectories in the Revised Hammersmith Scale in a Cohort of Untreated Patients with Spinal Muscular Atrophy types 2 and 3
- PMID: 38427497
- PMCID: PMC11091622
- DOI: 10.3233/JND-230211
Disease Trajectories in the Revised Hammersmith Scale in a Cohort of Untreated Patients with Spinal Muscular Atrophy types 2 and 3
Abstract
Background: Spinal muscular atrophy (SMA) is a neuromuscular disorder characterised by progressive motor function decline. Motor function is assessed using several functional outcome measures including the Revised Hammersmith Scale (RHS).
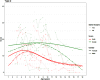
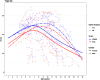
Objective: In this study, we present longitudinal trajectories for the RHS in an international cohort of 149 untreated paediatric SMA 2 and 3 patients (across 531 assessments collected between March 2015 and July 2019).
Methods: We contextualise these trajectories using both the Hammersmith Functional Motor Scale Expanded (HFMSE) and Revised Upper Limb Module (RULM). At baseline, this cohort included 50% females and 15% of patients had undergone spinal fusion surgery. Patient trajectories were modelled using a natural cubic spline with age, sex, and random effects for each patient.
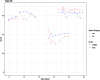
Results: RHS and HFMSE scores show similar trends over time in this cohort not receiving disease modifying therapies. The results confirm the strong correlation between the RHS and RULM previously observed in SMA types 2 and 3a. Scoliosis surgery is associated with a reduction of 3 points in the RHS, 4.5 points in the HFMSE for the SMA 2 population, and a reduction of 11.8 points in the RHS, and 13.4 points in the HFMSE for the SMA 3a populations. When comparing the RHS and RULM, there is a lower correlation in the type 3a's than the type 2 patients. In the SMA 2 population, there is no significant difference between the sexes in either the RHS or HFMSE trajectories. There is no significant difference in the RULM trajectory in the SMA 2 or 3a participants by sex.
Conclusions: This study demonstrates that the RHS could be used in conjunction with other functional measures such as the RULM to holistically detect SMA disease progression. This will assist with fully understanding changes that occur with treatments, further defining trajectories and therapy outcomes.
Keywords: Spinal muscular atrophy; outcome measure; physical therapists; scoliosis.
Conflict of interest statement
A.D. has received compensation as a consultant on advisory boards for Roche, Biogen, and AveXis. A.M. has served on medical/scientific advisory boards for Biogen and Roche, and has received fees for consulting and training services for Biogen, Roche, Novartis, and Biohaven. A.M.G. receives fees for consulting services for Biogen, Roche, and Audentes, and licensing fees for co-development of the CHOP INTEND. A.P. has served on medical/scientific advisory boards for AveXis, Biogen, and Roche, and has received fees for consulting services for Biogen, Roche, and Audentes. B.T.D. has served as an ad hoc scientific advisory board member for Audentes, AveXis/Novartis Gene Therapies, Biogen, Pfizer, Sarepta, Vertex, and Roche/Genentech; Steering Committee Chair for Roche FIREFISH and MANATEE studies, and DSMB member for Amicus Inc. and Lexeo Therapeutics; B.T.D. has no financial interests in these companies. B.T.D. has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Slaney Family Fund for SMA, the Spinal Muscular Atrophy Foundation, CureSMA, and Working on Walking Fund, and has received grants from Ionis Pharmaceuticals, Inc. for the ENDEAR, CHERISH, CS2/CS12 studies; from Biogen for CS11; and from AveXis, Sarepta Pharmaceuticals, Novartis (AveXis), PTC Therapeutics, Roche, Scholar Rock, and Fibrogen. B.T.D. has also received royalties for books and online publications from Elsevier and UpToDate, Inc. C.B. has served as a consultant, speaker in sponsored symposiums, and principal investigator for Roche, and has received personal fees for AveXis and Roche. C.M. has received consultancy honoraria from Biogen, Roche, and Novartis for participation in educational activities/meetings. C.M. has also received research funding from Roche and Biogen to support Adult SMA REACH activity. D.C.D.V has served as an advisor/consultant for AveXis, Biogen, Cytokinetics, Ionis, METAFORA, Roche, Sanofi, Sarepta, Scholar Rock, SMA Foundation, and Ultragenyx, with no financial interests in these companies; has received grants from Cure SMA, Department of Defense, Glut1 Deficiency Foundation, Hope for Children Research Foundation, National Institutes of Health, and SMA Foundation; has received research funding from Department of Defense, Glut1 Deficiency Foundation, Hope for Children Research Foundation, iSMAC initiative (Biogen), National Institutes of Health, Sanofi, and SMA Foundation; has received clinical trial funding from Ionis, Mallinckrodt, PTC, Santhera, Sarepta, Scholar Rock, and Ultragenyx; serves as the Data Safety Monitoring Committee Chair for Aspa Therapeutics; and is an inventor on a patent for Glut1DS gene therapy. D.R. reports participation to teaching initiatives for Roche. E.A. has served as a consultant and as a speaker in sponsored symposiums for Biogen. E.B. reports participation to Scientific Advisory Boards for Roche, Biogen, PTC Therapeutics, Pfizer, and Novartis. E.B. is involved in clinical trials with Roche, Biogen, Novartis, and PTC Therapeutics. E.Mercuri has participated in advisory boards for SMA studies for AveXis, Biogen, Ionis, Novartis, and Roche; has been a Principal Investigator for ongoing Biogen and Roche clinical trials; and has received research grants from Famiglie SMA Italy, Italian Telethon, Novartis, Scholar Rock, and SMA Europe. E.S.M. reports consultancy fees from Roche, Biogen, Scholar Rock, and Novartis. F.M. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, Roche, and Novartis. E.S.M. is a member of the Rare Disease Scientific Advisory Board for Pfizer. E.S.M. is involved as an investigator in clinical trials from AveXis, Biogen, and Roche. E.S.M. is the principal investigator of the SMA REACH UK clinical network, partially funded by Biogen and by SMA UK. G.B. is principal investigator of clinical trials sponsored by Pfizer, NS Pharma, and Reveragen; has received speaker and/or consulting fees from Sarepta, PTC Therapeutics, Biogen, Novartis Gene Therapies, Inc. (AveXis), and Roche; and has worked as principal investigator of SMA studies sponsored by Novartis Gene Therapies, Inc., and Roche. G.C. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, Roche, and Novartis. J.D. has received personal fees for AveXis, Biogen, Roche, and Novartis. J.M. serves on advisory boards for Biogen, Roche, Genentech, and Sarepta and as a consultant for Scholar Rock and Biogen. She receives grant support from the Muscular Dystrophy Association, Cure SMA, Genentech, and Novartis. M.P. has served as a consultant and as a speaker in sponsored symposiums for Biogen, and has received personal fees for AveXis. M.S. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, and Roche; M.S. is involved as an investigator in clinical trials from AveXis, Biogen, and Roche. R.M.L. has served in advisory boards for Biogen, Roche, and Novartis; has received consulting fees by Roche, Biogen, and Novartis; and research support by Roche and Biogen. R.S.F. has served on medical/scientific advisory boards on SMA-related topics for AveXis, Biogen, Capricor, Families of SMA, Genentech, Ionis Pharmaceuticals, Novartis, Roche, and ScholarRock; received research support from AveXis/Novartis, Biogen/Ionis, Capricor, Roche/Genentech, Scholar Rock, and NIH; and receives license fees for co-development of the CHOP INTEND. S.D.Y. serves on advisory boards for Biogen, Roche, and Scholar Rock, and as a consultant for Biogen, Roche, and Cure SMA. S.D.Y. receives grant support from Cure SMA. S.M. has received honoraria from Biogen, Roche, and Novartis for advisory boards and presentations. T.D. has served on scientific advisory boards for AveXis, Biogen, Roche, Scholar Rock, Novartis, CureSMA, Dyne, and Actigraph; and has received consulting fees Roche, Biogen, Biohaven, Dyne, Sarepta, Solid Bio, Tayjus, Astellas, ATOM, and Trinds. V.S. has served as a consultant and as a speaker in sponsored symposiums for Biogen, and has received personal fees for AveXis. A.W., E.O., E.Milev, G.S., A.R., M.C., M.M., and Z.Z.C. have no conflicting interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
2-Year Change in Revised Hammersmith Scale Scores in a Large Cohort of Untreated Paediatric Type 2 and 3 SMA Participants.J Clin Med. 2023 Feb 28;12(5):1920. doi: 10.3390/jcm12051920. J Clin Med. 2023. PMID: 36902710 Free PMC article.
-
Effectiveness of Nusinersen in Adolescents and Adults with Spinal Muscular Atrophy: Systematic Review and Meta-analysis.Neurol Ther. 2024 Oct;13(5):1483-1504. doi: 10.1007/s40120-024-00653-2. Epub 2024 Sep 2. Neurol Ther. 2024. PMID: 39222296 Free PMC article.
-
Efficacy and safety of Nusinersen among children with spinal muscular atrophy from North India: A prospective cohort study (NICE-SMA study).Eur J Paediatr Neurol. 2025 Jan;54:42-49. doi: 10.1016/j.ejpn.2024.12.001. Epub 2024 Dec 14. Eur J Paediatr Neurol. 2025. PMID: 39675174
-
Drug treatment for spinal muscular atrophy types II and III.Cochrane Database Syst Rev. 2020 Jan 6;1(1):CD006282. doi: 10.1002/14651858.CD006282.pub5. Cochrane Database Syst Rev. 2020. PMID: 32006461 Free PMC article.
-
Nusinersen in Adults with 5q Spinal Muscular Atrophy: a Systematic Review and Meta-analysis.Neurotherapeutics. 2022 Mar;19(2):464-475. doi: 10.1007/s13311-022-01200-3. Epub 2022 Feb 17. Neurotherapeutics. 2022. PMID: 35178673 Free PMC article.
Cited by
-
Scoliosis in spinal muscular atrophy in the era of disease-modifying therapy: a scoping review.Neurol Sci. 2025 Aug;46(8):3431-3442. doi: 10.1007/s10072-025-08155-1. Epub 2025 Apr 2. Neurol Sci. 2025. PMID: 40172735 Free PMC article.
-
Long-term natural history in type II and III spinal muscular atrophy: a 4-year international study on the Hammersmith Functional Motor Scale Expanded.Eur J Neurol. 2024 Dec;31(12):e16517. doi: 10.1111/ene.16517. Epub 2024 Oct 11. Eur J Neurol. 2024. PMID: 39392101 Free PMC article.
-
Correlation Between the Motor Outcomes and SMN2 and NAIP Gene Copy Numbers Among North Indian Children with Spinal Muscular Atrophy.Ann Indian Acad Neurol. 2025 Jul 1;28(4):579-584. doi: 10.4103/aian.aian_974_24. Epub 2025 Jun 3. Ann Indian Acad Neurol. 2025. PMID: 40820838 Free PMC article.
-
Upper limb motor function in individuals with SMA type 2: natural history and impact of therapies.J Neurol. 2025 Apr 9;272(5):331. doi: 10.1007/s00415-025-13042-y. J Neurol. 2025. PMID: 40205228
References
-
- Petit F, Cuisset JM, Rouaix-Emery N, Cancés C, Sablonnière B, Bieth E, et al.. Insights into genotype-phenotype correlations in spinal muscular atrophy: A retrospective study of 103 patients. Muscle Nerve. 2011;43(1):26–30. - PubMed
-
- Piepers S, Van Den Berg LH, Brugman F, Scheffer H, Ruiterkamp-Versteeg M, Van Engelen BG, Faber CG, De Visser M, van der Pol WL, Wokke JHJ A natural history study of late onset spinal muscular atrophy types 3b and 4. Journal of Neurology. 2008;255:1400–4. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

