Platelet dysfunction reversal with cold-stored vs room temperature-stored platelet transfusions
- PMID: 38427589
- PMCID: PMC11143524
- DOI: 10.1182/blood.2023022593
Platelet dysfunction reversal with cold-stored vs room temperature-stored platelet transfusions
Abstract
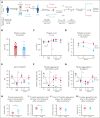
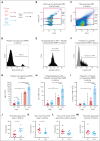
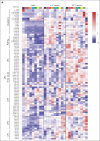
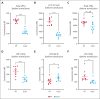
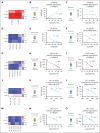
Platelets are stored at room temperature for 5 to 7 days (room temperature-stored platelets [RSPs]). Because of frequent and severe shortages, the US Food and Drug Administration recently approved up to 14-day cold-stored platelets (CSPs) in plasma. However, the posttransfusion function of CSPs is unknown and it is unclear which donors are best suited to provide either RSPs or CSPs. In this study, we sought to evaluate the posttransfusion platelet function and its predictors for platelets stored for the maximum approved storage times (7-day RSPs and 14-day CSPs) in healthy volunteers on acetylsalicylic acid (ASA). We conducted a randomized crossover study in 10 healthy humans. Individuals donated 1 platelet unit, stored at either 22°C or 4°C based on randomization. Before transfusion, participants ingested ASA to inhibit endogenous platelets. Transfusion recipients were tested for platelet function and lipid mediators. Platelet units were tested for lipid mediators only. A second round of transfusion with the alternative product was followed by an identical testing sequence. RSPs reversed platelet inhibition significantly better in αIIbβ3 integrin activation-dependent assays. In contrast, CSPs in recipients led to significantly more thrombin generation, which was independent of platelet microparticles. Lysophosphatidylcholine-O species levels predicted the procoagulant capacity of CSPs. In contrast, polyunsaturated fatty acid concentrations predicted the aggregation response of RSPs. In summary, we provide, to our knowledge, the first efficacy data of extended-stored CSPs in plasma. Our results suggest that identifying ideal RSP and CSP donors is possible, and pave the way for larger studies in the future. This trial is registered at www.ClinicalTrials.gov as #NCT0511102.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. has received research support from Terumo Blood Component Technologies and Cerus. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Platelets: let's chill until more data arrive.Blood. 2024 May 16;143(20):2019-2020. doi: 10.1182/blood.2024024329. Blood. 2024. PMID: 38753356 No abstract available.
References
-
- Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. 2019;380(3):242–251. - PubMed
-
- Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–2613. - PubMed
-
- Cap AP. Platelet storage: a license to chill! Transfusion. 2016;56(1):13–16. - PubMed
-
- Reddoch-Cardenas KM, Peltier GC, Chance TC, et al. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion. 2021;61(1):178–190. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

