Polymorph Identification for Flexible Molecules: Linear Regression Analysis of Experimental and Calculated Solution- and Solid-State NMR Data
- PMID: 38427685
- PMCID: PMC10945485
- DOI: 10.1021/acs.jpca.3c07732
Polymorph Identification for Flexible Molecules: Linear Regression Analysis of Experimental and Calculated Solution- and Solid-State NMR Data
Abstract
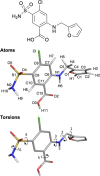
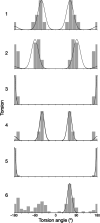
The Δδ regression approach of Blade et al. [ J. Phys. Chem. A 2020, 124(43), 8959-8977] for accurately discriminating between solid forms using a combination of experimental solution- and solid-state NMR data with density functional theory (DFT) calculation is here extended to molecules with multiple conformational degrees of freedom, using furosemide polymorphs as an exemplar. As before, the differences in measured 1H and 13C chemical shifts between solution-state NMR and solid-state magic-angle spinning (MAS) NMR (Δδexperimental) are compared to those determined by gauge-including projector augmented wave (GIPAW) calculations (Δδcalculated) by regression analysis and a t-test, allowing the correct furosemide polymorph to be precisely identified. Monte Carlo random sampling is used to calculate solution-state NMR chemical shifts, reducing computation times by avoiding the need to systematically sample the multidimensional conformational landscape that furosemide occupies in solution. The solvent conditions should be chosen to match the molecule's charge state between the solution and solid states. The Δδ regression approach indicates whether or not correlations between Δδexperimental and Δδcalculated are statistically significant; the approach is differently sensitive to the popular root mean squared error (RMSE) method, being shown to exhibit a much greater dynamic range. An alternative method for estimating solution-state NMR chemical shifts by approximating the measured solution-state dynamic 3D behavior with an ensemble of 54 furosemide crystal structures (polymorphs and cocrystals) from the Cambridge Structural Database (CSD) was also successful in this case, suggesting new avenues for this method that may overcome its current dependency on the prior determination of solution dynamic 3D structures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Conformations in Solution and in Solid-State Polymorphs: Correlating Experimental and Calculated Nuclear Magnetic Resonance Chemical Shifts for Tolfenamic Acid.J Phys Chem A. 2020 Oct 29;124(43):8959-8977. doi: 10.1021/acs.jpca.0c07000. Epub 2020 Oct 16. J Phys Chem A. 2020. PMID: 32946236
-
An NMR crystallography investigation of furosemide.Magn Reson Chem. 2019 May;57(5):191-199. doi: 10.1002/mrc.4789. Epub 2018 Oct 11. Magn Reson Chem. 2019. PMID: 30141257 Free PMC article.
-
The use of variable temperature 13 C solid-state MAS NMR and GIPAW DFT calculations to explore the dynamics of diethylcarbamazine citrate.Magn Reson Chem. 2019 May;57(5):200-210. doi: 10.1002/mrc.4790. Epub 2018 Oct 15. Magn Reson Chem. 2019. PMID: 30114322
-
Proton-Based Ultrafast Magic Angle Spinning Solid-State NMR Spectroscopy.Acc Chem Res. 2017 Apr 18;50(4):1105-1113. doi: 10.1021/acs.accounts.7b00082. Epub 2017 Mar 29. Acc Chem Res. 2017. PMID: 28353338 Free PMC article. Review.
-
Solid-state NMR structures of integral membrane proteins.Mol Membr Biol. 2015 Aug-Dec;32(5-8):156-78. doi: 10.3109/09687688.2016.1139754. Epub 2016 Feb 8. Mol Membr Biol. 2015. PMID: 26857803 Review.
Cited by
-
Exploring the crystallisation of aspirin in a confined porous material using solid-state nuclear magnetic resonance.Faraday Discuss. 2025 Jan 8;255(0):483-494. doi: 10.1039/d4fd00123k. Faraday Discuss. 2025. PMID: 39356059 Free PMC article.
-
Organic NMR crystallography: enabling progress for applications to pharmaceuticals and plant cell walls.Faraday Discuss. 2025 Jan 8;255(0):222-243. doi: 10.1039/d4fd00088a. Faraday Discuss. 2025. PMID: 39600178 Free PMC article.
References
-
- Bhardwaj R. M.; McMahon J. A.; Nyman J.; Price L. S.; Konar S.; Oswald I. D. H.; Pulham C. R.; Price S. L.; Reutzel-Edens S. M. A Prolific Solvate Former, Galunisertib, under the Pressure of Crystal Structure Prediction, Produces Ten Diverse Polymorphs. J. Am. Chem. Soc. 2019, 141 (35), 13887–13897. 10.1021/jacs.9b06634. - DOI - PubMed
-
- Mortazavi M.; Hoja J.; Aerts L.; Quéré L.; van de Streek J.; Neumann M. A.; Tkatchenko A. Computational polymorph screening reveals late-appearing and poorly-soluble form of rotigotine. Commun. Chem. 2019, 2 (1), 7010.1038/s42004-019-0171-y. - DOI
-
- Greenwell C.; McKinley J. L.; Zhang P.; Zeng Q.; Sun G.; Li B.; Wen S.; Beran G. J. O. Overcoming the difficulties of predicting conformational polymorph energetics in molecular crystals via correlated wavefunction methods. Chem. Sci. 2020, 11 (8), 2200–2214. 10.1039/C9SC05689K. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

