Computational models of compound nerve action potentials: Efficient filter-based methods to quantify effects of tissue conductivities, conduction distance, and nerve fiber parameters
- PMID: 38427699
- PMCID: PMC10936855
- DOI: 10.1371/journal.pcbi.1011833
Computational models of compound nerve action potentials: Efficient filter-based methods to quantify effects of tissue conductivities, conduction distance, and nerve fiber parameters
Abstract
Background: Peripheral nerve recordings can enhance the efficacy of neurostimulation therapies by providing a feedback signal to adjust stimulation settings for greater efficacy or reduced side effects. Computational models can accelerate the development of interfaces with high signal-to-noise ratio and selective recording. However, validation and tuning of model outputs against in vivo recordings remains computationally prohibitive due to the large number of fibers in a nerve.
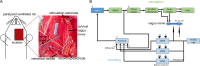
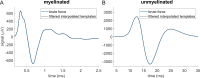
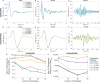
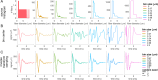
Methods: We designed and implemented highly efficient modeling methods for simulating electrically evoked compound nerve action potential (CNAP) signals. The method simulated a subset of fiber diameters present in the nerve using NEURON, interpolated action potential templates across fiber diameters, and filtered the templates with a weighting function derived from fiber-specific conduction velocity and electromagnetic reciprocity outputs of a volume conductor model. We applied the methods to simulate CNAPs from rat cervical vagus nerve.
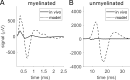
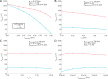
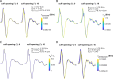
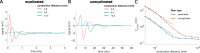
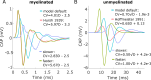
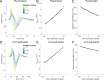
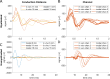
Results: Brute force simulation of a rat vagal CNAP with all 1,759 myelinated and 13,283 unmyelinated fibers in NEURON required 286 and 15,860 CPU hours, respectively, while filtering interpolated templates required 30 and 38 seconds on a desktop computer while maintaining accuracy. Modeled CNAP amplitude could vary by over two orders of magnitude depending on tissue conductivities and cuff opening within experimentally relevant ranges. Conduction distance and fiber diameter distribution also strongly influenced the modeled CNAP amplitude, shape, and latency. Modeled and in vivo signals had comparable shape, amplitude, and latency for myelinated fibers but not for unmyelinated fibers.
Conclusions: Highly efficient methods of modeling neural recordings quantified the large impact that tissue properties, conduction distance, and nerve fiber parameters have on CNAPs. These methods expand the computational accessibility of neural recording models, enable efficient model tuning for validation, and facilitate the design of novel recording interfaces for neurostimulation feedback and understanding physiological systems.
Copyright: © 2024 Peña et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Waveform analysis of compound nerve action potentials: a computer simulation.Arch Phys Med Rehabil. 1994 Sep;75(9):960-4. Arch Phys Med Rehabil. 1994. PMID: 8085930
-
Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fiber input to brainstem nuclei.Exp Brain Res. 1998 Aug;121(4):379-90. doi: 10.1007/s002210050472. Exp Brain Res. 1998. PMID: 9746144
-
Computational modelling of nerve stimulation and recording with peripheral visceral neural interfaces.J Neural Eng. 2021 Nov 25;18(6):10.1088/1741-2552/ac36e2. doi: 10.1088/1741-2552/ac36e2. J Neural Eng. 2021. PMID: 34740201 Free PMC article.
-
[Test for analysing nerve conduction velocity].Rinsho Shinkeigaku. 1991 Dec;31(12):1326-9. Rinsho Shinkeigaku. 1991. PMID: 1817800 Review. Japanese.
-
Models and analysis of compound nerve action potentials.Crit Rev Biomed Eng. 1991;19(1):47-111. Crit Rev Biomed Eng. 1991. PMID: 1893755 Review.
Cited by
-
Computational modeling of autonomic nerve stimulation: Vagus et al.Curr Opin Biomed Eng. 2024 Dec;32:100557. doi: 10.1016/j.cobme.2024.100557. Epub 2024 Aug 24. Curr Opin Biomed Eng. 2024. PMID: 39650310
-
Biophysical characterization of the recording of unmyelinated and myelinated fiber activity with peripheral interfaces.iScience. 2025 Apr 22;28(5):112495. doi: 10.1016/j.isci.2025.112495. eCollection 2025 May 16. iScience. 2025. PMID: 40458190 Free PMC article.
-
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies.PLoS Comput Biol. 2024 Jul 12;20(7):e1011826. doi: 10.1371/journal.pcbi.1011826. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38995970 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

