The FLRT3-UNC5B checkpoint pathway inhibits T cell-based cancer immunotherapies
- PMID: 38427724
- PMCID: PMC10906930
- DOI: 10.1126/sciadv.adj4698
The FLRT3-UNC5B checkpoint pathway inhibits T cell-based cancer immunotherapies
Abstract
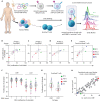
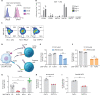
Cancers exploit coinhibitory receptors on T cells to escape tumor immunity, and targeting such mechanisms has shown remarkable clinical benefit, but in a limited subset of patients. We hypothesized that cancer cells mimic noncanonical mechanisms of early development such as axon guidance pathways to evade T cell immunity. Using gain-of-function genetic screens, we profiled axon guidance proteins on human T cells and their cognate ligands and identified fibronectin leucine-rich transmembrane protein 3 (FLRT3) as a ligand that inhibits T cell activity. We demonstrated that FLRT3 inhibits T cells through UNC5B, an axon guidance receptor that is up-regulated on activated human T cells. FLRT3 expressed in human cancers favored tumor growth and inhibited CAR-T and BiTE + T cell killing and infiltration in humanized cancer models. An FLRT3 monoclonal antibody that blocked FLRT3-UNC5B interactions reversed these effects in an immune-dependent manner. This study supports the concept that axon guidance proteins mimic T cell checkpoints and can be targeted for cancer immunotherapy.
Figures





References
-
- Hosseinkhani N., Derakhshani A., Kooshkaki O., Abdoli Shadbad M., Hajiasgharzadeh K., Baghbanzadeh A., Safarpour H., Mokhtarzadeh A., Brunetti O., Yue S., Silvestris N., Baradaran B., Immune checkpoints and CAR-T cells: The pioneers in future cancer therapies? Int. J. Mol. Sci. 21, 8305 (2020). - PMC - PubMed
-
- FDA approves anti-LAG3 checkpoint. Nat. Biotechnol. 40, 625 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

