Aberrant RNA sensing in regulatory T cells causes systemic autoimmunity
- PMID: 38427731
- PMCID: PMC10906915
- DOI: 10.1126/sciadv.adk0820
Aberrant RNA sensing in regulatory T cells causes systemic autoimmunity
Abstract
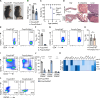
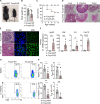
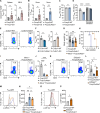
Chronic and aberrant nucleic acid sensing causes type I IFN-driven autoimmune diseases, designated type I interferonopathies. We found a significant reduction of regulatory T cells (Tregs) in patients with type I interferonopathies caused by mutations in ADAR1 or IFIH1 (encoding MDA5). We analyzed the underlying mechanisms using murine models and found that Treg-specific deletion of Adar1 caused peripheral Treg loss and scurfy-like lethal autoimmune disorders. Similarly, knock-in mice with Treg-specific expression of an MDA5 gain-of-function mutant caused apoptosis of peripheral Tregs and severe autoimmunity. Moreover, the impact of ADAR1 deficiency on Tregs is multifaceted, involving both MDA5 and PKR sensing. Together, our results highlight the dysregulation of Treg homeostasis by intrinsic aberrant RNA sensing as a potential determinant for type I interferonopathies.
Figures

References
-
- Lee-Kirsch M. A., The type I interferonopathies. Annu. Rev. Med. 68, 297–315 (2017). - PubMed
-
- Crow Y. J., Hayward B. E., Parmar R., Robins P., Leitch A., Ali M., Black D. N., van Bokhoven H., Brunner H. G., Hamel B. C., Corry P. C., Cowan F. M., Frints S. G., Klepper J., Livingston J. H., Lynch S. A., Massey R. F., Meritet J. F., Michaud J. L., Ponsot G., Voit T., Lebon P., Bonthron D. T., Jackson A. P., Barnes D. E., Lindahl T., Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006). - PubMed
-
- Rice G. I., Bond J., Asipu A., Brunette R. L., Manfield I. W., Carr I. M., Fuller J. C., Jackson R. M., Lamb T., Briggs T. A., Ali M., Gornall H., Couthard L. R., Aeby A., Attard-Montalto S. P., Bertini E., Bodemer C., Brockmann K., Brueton L. A., Corry P. C., Desguerre I., Fazzi E., Cazorla A. G., Gener B., Hamel B. C. J., Heiberg A., Hunter M., van der Knaap M. S., Kumar R., Lagae L., Landrieu P. G., Lourenco C. M., Marom D., McDermott M. F., van der Merwe W., Orcesi S., Prendiville J. S., Rasmussen M., Shalev S. A., Soler D. M., Shinawi M., Spiegel R., Tan T. Y., Vanderver A., Wakeling E. L., Wassmer E., Whittaker E., Lebon P., Stetson D. B., Bonthron D. T., Crow Y. J., Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009). - PMC - PubMed
-
- Crow Y. J., Leitch A., Hayward B. E., Garner A., Parmar R., Griffith E., Ali M., Semple C., Aicardi J., Babul-Hirji R., Baumann C., Baxter P., Bertini E., Chandler K. E., Chitayat D., Cau D., Déry C., Fazzi E., Goizet C., King M. D., Klepper J., Lacombe D., Lanzi G., Lyall H., Martínez-Frías M. L., Mathieu M., McKeown C., Monier A., Oade Y., Quarrell O. W., Rittey C. D., Rogers R. C., Sanchis A., Stephenson J. B. P., Tacke U., Till M., Tolmie J. L., Tomlin P., Voit T., Weschke B., Woods C. G., Lebon P., Bonthron D. T., Ponting C. P., Jackson A. P., Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

