Early IGF-1 receptor inhibition in mice mimics preterm human brain disorders and reveals a therapeutic target
- PMID: 38427732
- PMCID: PMC10906931
- DOI: 10.1126/sciadv.adk8123
Early IGF-1 receptor inhibition in mice mimics preterm human brain disorders and reveals a therapeutic target
Abstract
Besides recent advances in neonatal care, preterm newborns still develop sex-biased behavioral alterations. Preterms fail to receive placental insulin-like growth factor-1 (IGF-1), a major fetal growth hormone in utero, and low IGF-1 serum levels correlate with preterm poor neurodevelopmental outcomes. Here, we mimicked IGF-1 deficiency of preterm newborns in mice by perinatal administration of an IGF-1 receptor antagonist. This resulted in sex-biased brain microstructural, functional, and behavioral alterations, resembling those of ex-preterm children, which we characterized performing parallel mouse/human behavioral tests. Pharmacological enhancement of GABAergic tonic inhibition by the U.S. Food and Drug Administration-approved drug ganaxolone rescued functional/behavioral alterations in mice. Establishing an unprecedented mouse model of prematurity, our work dissects the mechanisms at the core of abnormal behaviors and identifies a readily translatable therapeutic strategy for preterm brain disorders.
Figures

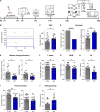
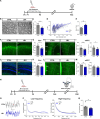
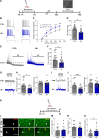
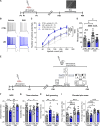
References
-
- Fernandez-Baizan C., Alcantara-Canabal L., Solis G., Mendez M., The association between perinatal and neonatal variables and neuropsychological development in very and extremely low-birth-weight preterm children at the beginning of primary school. Appl. Neuropsychol. Child 10, 348–358 (2021). - PubMed
-
- Johnson S., Hollis C., Kochhar P., Hennessy E., Wolke D., Marlow N., Psychiatric disorders in extremely preterm children: Longitudinal finding at age 11 years in the EPICure study. J. Am. Acad. Child Adolesc. Psychiatry 49, 453–463.e1 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

