Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells
- PMID: 38428412
- PMCID: PMC11003464
- DOI: 10.1016/j.ccell.2024.02.004
Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells
Abstract
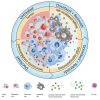
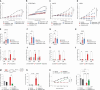
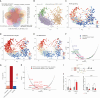
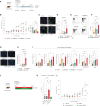
Cellular senescence can exert dual effects in tumors, either suppressing or promoting tumor progression. The senescence-associated secretory phenotype (SASP), released by senescent cells, plays a crucial role in this dichotomy. Consequently, the clinical challenge lies in developing therapies that safely enhance senescence in cancer, favoring tumor-suppressive SASP factors over tumor-promoting ones. Here, we identify the retinoic-acid-receptor (RAR) agonist adapalene as an effective pro-senescence compound in prostate cancer (PCa). Reactivation of RARs triggers a robust senescence response and a tumor-suppressive SASP. In preclinical mouse models of PCa, the combination of adapalene and docetaxel promotes a tumor-suppressive SASP that enhances natural killer (NK) cell-mediated tumor clearance more effectively than either agent alone. This approach increases the efficacy of the allogenic infusion of human NK cells in mice injected with human PCa cells, suggesting an alternative therapeutic strategy to stimulate the anti-tumor immune response in "immunologically cold" tumors.
Keywords: AP-1; NK-Killing; RAR; SASP; adapalene; allogenic infusion; immunotherapy; metabolism; prostate cancer; senescence.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.A. is a co-founder of and owns stock in OncoSense, and A.A., M.C., and A.R. are inventors of the patent WO2019142095A1 (Title: new alk inhibitor senolytic drugs).
Figures



References
-
- Petrylak D.P., Tangen C.M., Hussain M.H.A., Lara P.N., Jr., Jones J.A., Taplin M.E., Burch P.A., Berry D., Moinpour C., Kohli M., et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

