Maternal inflammation regulates fetal emergency myelopoiesis
- PMID: 38428422
- PMCID: PMC10954379
- DOI: 10.1016/j.cell.2024.02.002
Maternal inflammation regulates fetal emergency myelopoiesis
Abstract
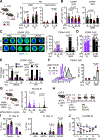
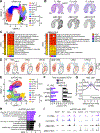
Neonates are highly susceptible to inflammation and infection. Here, we investigate how late fetal liver (FL) mouse hematopoietic stem and progenitor cells (HSPCs) respond to inflammation, testing the hypothesis that deficits in the engagement of emergency myelopoiesis (EM) pathways limit neutrophil output and contribute to perinatal neutropenia. We show that fetal HSPCs have limited production of myeloid cells at steady state and fail to activate a classical adult-like EM transcriptional program. Moreover, we find that fetal HSPCs can respond to EM-inducing inflammatory stimuli in vitro but are restricted by maternal anti-inflammatory factors, primarily interleukin-10 (IL-10), from activating EM pathways in utero. Accordingly, we demonstrate that the loss of maternal IL-10 restores EM activation in fetal HSPCs but at the cost of fetal demise. These results reveal the evolutionary trade-off inherent in maternal anti-inflammatory responses that maintain pregnancy but render the fetus unresponsive to EM activation signals and susceptible to infection.
Keywords: developmental hematopoiesis; emergency myelopoiesis; fetus; hematopoietic stem cells; interleukin-10; maternal-fetal crosstalk; multipotent progenitors; neonate; neutropenia.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Update of
-
Maternal IL-10 restricts fetal emergency myelopoiesis.bioRxiv [Preprint]. 2023 Sep 13:2023.09.13.557548. doi: 10.1101/2023.09.13.557548. bioRxiv. 2023. Update in: Cell. 2024 Mar 14;187(6):1402-1421.e21. doi: 10.1016/j.cell.2024.02.002. PMID: 37745377 Free PMC article. Updated. Preprint.
References
-
- Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, Weichenhan D, Lier A, von Paleske L, Renders S, et al. (2014). Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 15, 507–522. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

