Therapeutic benefit of idebenone in patients with Leber hereditary optic neuropathy: The LEROS nonrandomized controlled trial
- PMID: 38428428
- PMCID: PMC10982982
- DOI: 10.1016/j.xcrm.2024.101437
Therapeutic benefit of idebenone in patients with Leber hereditary optic neuropathy: The LEROS nonrandomized controlled trial
Abstract
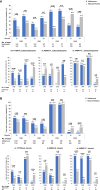
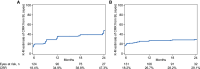
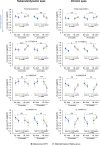
Leber hereditary optic neuropathy (LHON) is a mitochondrial disease leading to rapid and severe bilateral vision loss. Idebenone has been shown to be effective in stabilizing and restoring vision in patients treated within 1 year of onset of vision loss. The open-label, international, multicenter, natural history-controlled LEROS study (ClinicalTrials.gov NCT02774005) assesses the efficacy and safety of idebenone treatment (900 mg/day) in patients with LHON up to 5 years after symptom onset (N = 199) and over a treatment period of 24 months, compared to an external natural history control cohort (N = 372), matched by time since symptom onset. LEROS meets its primary endpoint and confirms the long-term efficacy of idebenone in the subacute/dynamic and chronic phases; the treatment effect varies depending on disease phase and the causative mtDNA mutation. The findings of the LEROS study will help guide the clinical management of patients with LHON.
Keywords: LHON; Leber hereditary optic neuropathy; idebenone; mitochondrial disease; mtDNA; neuro-ophthalmology; optic atrophy; optic neuropathy; retinal ganglion cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.Y.-W.-M., V.C., N.J.N., B.P., L.C., and T.K. received research support and/or personal compensation from Santhera Pharmaceuticals, Chiesi, and GenSight Biologics. P.Y.-W.-M. is also a consultant for Neurophth and Stoke Therapeutics. N.J.N. is also a consultant for Santhera Pharmaceutical, Chiesi, GenSight Biologics, Stoke Therapeutics, Avidity Biosciences, and Neurophoenix SAS, and has been a speaker and contributing writer for educational activities under the auspices of WebMD and First Class. P.S.S. is a consultant to GenSight Biologics and received research support from GenSight Biologics and Santhera Pharmaceuticals. M.H.L. received personal compensation from Santhera Pharmaceuticals. C.L.M. has acted as a consultant for Chiesi Farmaceutici, Regulatory PharmaNet, and Thenewway srl, and has also received speaker honoraria and/or financial support for meetings from these companies, as well as from First Class srl and Biologix. C.L.M. has also acted as a principal or study investigator for clinical trials sponsored by GenSight Biologics, Santhera Pharmaceuticals, Stoke Therapeutics, and Reneo Pharmaceuticals. C.P. received personal compensation from Novartis and has acted as an investigator in clinical trials for Santhera Pharmaceuticals, GenSight Biologics, and Iveric Bio. X.L. and L.T. are employees of Chiesi Farmaceutici S.p.A.
Figures


References
-
- Carelli V., Carbonelli M., de Coo I.F., Kawasaki A., Klopstock T., Lagrèze W.A., La Morgia C., Newman N.J., Orssaud C., Pott J.W.R., et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J. Neuro Ophthalmol. 2017;37:371–381. doi: 10.1097/WNO.0000000000000570. - DOI - PubMed
-
- Gueven N. Optic neurodegeneration: Time to act. Biol. Med. 2014;01 doi: 10.4172/0974-8369.S1-001. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

