OPTIMA-BP: empOwering PaTients in MAnaging Blood Pressure - protocol for a randomised parallel group study comparing use of Kvatchii web-based patient education portal as an addition to home blood pressure monitoring
- PMID: 38429056
- PMCID: PMC10910568
- DOI: 10.1136/openhrt-2023-002535
OPTIMA-BP: empOwering PaTients in MAnaging Blood Pressure - protocol for a randomised parallel group study comparing use of Kvatchii web-based patient education portal as an addition to home blood pressure monitoring
Abstract
Introduction: Hypertension is the leading modifiable risk factor for cardiovascular disease and is implicated in half of all strokes and myocardial infarctions. One-third of the adults in Scotland have hypertension yet only a quarter of them have their blood pressure (BP) controlled to target (<140/90 mm Hg). Empowering patients to have a better understanding of their condition and becoming actively involved in the monitoring and management of hypertension may lead to improved patient satisfaction, improved BP control and health outcomes and reduction in the use of primary/secondary care hypertension clinics.
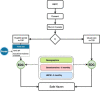
Methods and analysis: OPTIMA-BP is a randomised parallel group pilot study comparing the use of home BP monitoring accompanied by access to the web-based cardiovascular educational portal (Kvatchii) and home BP monitoring (HBPM) alone in 200 patients with hypertension attending the Glasgow Blood Pressure Clinic, Queen Elizabeth University Hospital, Glasgow. Consented participants will be asked to complete surveys on lifestyle factors, medication adherence, quality of life and hypertension knowledge, understanding and home monitoring. The intervention group will be asked to complete a survey to help evaluate the Kvatchii portal. At 6 and 12 months, the surveys will be repeated via the CASTOR EDC. Both groups will input their HBPM results at 2-month intervals into a CASTOR-EDC survey. OPTIMA-BP will follow-up with participants over 12 months with the study running over 24 months. The primary outcome is HBPM systolic BP area under the curve between baseline and 6 months ETHICS AND DISSEMINATION: OPTIMA-BP was approved by the North of Scotland Research Ethics Committee 2 (22/NS/0095). Current protocol version 1.2 date 6 June 2023. Written informed consent will be provided by all study participants. Study findings will be submitted to international peer-reviewed journals and will be presented at national and international scientific meetings.
Trial registration number: ClinicalTrials.gov: NCT05575453. Registered 12 October 2022. https://clinicaltrials.gov/ct2/show/NCT05575453.
Keywords: Delivery of Health Care; Hypertension; Research Design.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SP is a Professor of Cardiovascular Genomics and Therapeutics at the University of Glasgow and an Honorary NHS Consultant Physician who works in the Glasgow Blood Pressure Clinic. SP is a cofounder and director of Kvatchii Limited and developed the education portal. There are no other competing interests.
Figures
Similar articles
-
Home and Online Management and Evaluation of Blood Pressure (HOME BP) digital intervention for self-management of uncontrolled, essential hypertension: a protocol for the randomised controlled HOME BP trial.BMJ Open. 2016 Nov 7;6(11):e012684. doi: 10.1136/bmjopen-2016-012684. BMJ Open. 2016. PMID: 27821598 Free PMC article. Clinical Trial.
-
Blood pressure monitoring in high-risk pregnancy to improve the detection and monitoring of hypertension (the BUMP 1 and 2 trials): protocol for two linked randomised controlled trials.BMJ Open. 2020 Jan 23;10(1):e034593. doi: 10.1136/bmjopen-2019-034593. BMJ Open. 2020. PMID: 31980512 Free PMC article. Clinical Trial.
-
Rationale and Design for the LOnger-term effects of SARS-CoV-2 INfection on blood Vessels And blood pRessure (LOCHINVAR): an observational phenotyping study.Open Heart. 2022 Jun;9(1):e002057. doi: 10.1136/openhrt-2022-002057. Open Heart. 2022. PMID: 35750422 Free PMC article.
-
The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan.Hypertens Res. 2013 Aug;36(8):661-72. doi: 10.1038/hr.2013.38. Epub 2013 Apr 18. Hypertens Res. 2013. PMID: 23595050 Review.
-
Research and Development of Information and Communication Technology-based Home Blood Pressure Monitoring from Morning to Nocturnal Hypertension.Ann Glob Health. 2016 Mar-Apr;82(2):254-73. doi: 10.1016/j.aogh.2016.02.004. Ann Glob Health. 2016. PMID: 27372530 Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous