Pooled analysis of pralatrexate single-agent studies in patients with relapsed/refractory peripheral T-cell lymphoma
- PMID: 38429077
- PMCID: PMC11157204
- DOI: 10.1182/bloodadvances.2023010441
Pooled analysis of pralatrexate single-agent studies in patients with relapsed/refractory peripheral T-cell lymphoma
Abstract
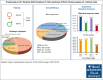
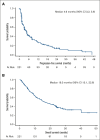
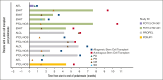
Patients with relapsed or refractory (R/R) mature natural killer cell and T-cell lymphoma have limited treatment options. To evaluate pralatrexate's performance and factors influencing its safety and efficacy in R/R peripheral T-cell lymphoma (PTCL), we performed a pooled analysis of data from 4 similarly designed, regulatory-mandated prospective clinical trials. Of 221 patients (median age, 59 years; 67.0% male) in the study population, 48.9% had PTCL not otherwise specified (PTCL-NOS), 21.3% angioimmunoblastic T-cell lymphoma, and 11.8% ALK-negative anaplastic large cell lymphoma (ALCL). Patients received pralatrexate for a median of 2.56 months (range, 0.03-24.18) and had a 40.7% objective response rate with a median duration of response of 9.1 months, progression-free survival 4.6 months, and overall survival 16.3 months. The most common treatment-related all-grade adverse events were stomatitis, thrombocytopenia, white blood cell count decrease, pyrexia, and vomiting. Subgroup exploratory analyses suggest improved efficacy with 1 prior line of chemotherapy vs 2 or ≥4 prior lines; PTCL-NOS or ALCL vs transformed mycosis fungoides; chemotherapy and transplant before pralatrexate vs chemotherapy alone or chemotherapy with other nontransplant treatments. In conclusion, these pooled analysis results further support using pralatrexate in patients with R/R PTCL. Prospective studies are needed to confirm the findings of subgroups analyses.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: O.A.O. has received research support from Merck and Astex; and is on the board of directors for Kymera, Myeloid Therapeutics, and Dren Pharmaceuticals, for which he receives an honorarium and is an equity holder. D.M. receives research funding from Astellas Pharma, Celgene, Novartis, Chugai, Ono, Takeda, Janssen, Sanofi, MSD, Otsuka, Eisai, AbbVie, Amgen, and BMS; and receives honoraria from Celgene, Chugai, Ono, Takeda, Janssen, Sanofi, MSD, Eisai, AbbVie, BMS, Mundipharma, Kyowa Kirin, Zenyaku, AstraZeneca, Nippon, and SymBio. E.-M.Y. is employed by Mundipharma Singapore Holdings Pte Ltd. N.M. is employed by Mundipharma Research Limited. K.T. receives honoraria from Celgene, Chugai, Eisai, Daiichi Sankyo, HUYA Bioscience International, Kyowa Kirin, Mundipharma, Ono Pharmaceutical, Solasia Pharma, Takeda, Yakult, and ZenyakuKogyo; and consults for Celgene, Daiichi Sankyo, HUYA Bioscience International, Mundipharma, Ono Pharmaceutical, Takeda, and Zenyaku Kogyo. The remaining authors declare no competing financial interests.
Figures


References
-
- Marchi E, O'Connor OA. The rapidly changing landscape in mature T-cell lymphoma (MTCL) biology and management. CA Cancer J Clin. 2020;70(1):47–70. - PubMed
-
- International T-Cell Lymphoma Project. Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. - PubMed
-
- Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970–1976. - PubMed

